Tetracaine (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tetracaine (injection) is a local anesthetic of the ester-linkage type, that is FDA approved for the procedure of spinal anesthesia for procedures requiring two to three hours. Common adverse reactions include nausea and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Tetracaine is indicated for the production of spinal anesthesia for procedures requiring two to three hours.
Dosage
- As with all anesthetics the dosage varies and depends upon the area to be anesthetized, the number of neuronal segments to be blocked, individual tolerance, and the technique of anesthesia. The lowest dosage needed to provide effective anesthesia should be administered. For specific techniques and procedures, refer to standard textbooks.

- The extent and degree of spinal anesthesia depend upon dosage, specific gravity of the anesthetic solution, volume of solution used, force of the injection, level of puncture, position of the patient during and immediately after injection, etc.
- When spinal fluid is added to either the NIPHANOID or the 1% Solution, some turbidity results, the degree depending on the pH of the spinal fluid, the temperature of the solution during mixing, as well as the amount of drug and diluent employed. This cloudiness is due to the release of the base from the hydrochloride. Liberation of base (which is completed within the spinal canal) is held to be essential for satisfactory results with any spinal anesthetic.
- The specific gravity of spinal fluid at 25°C/25°C varies under normal conditions from 1.0063 to 1.0075. A solution of the instantly soluble form (NIPHANOID) in spinal fluid has only a slightly greater specific gravity. The 1% concentration in saline solution has a specific gravity of 1.0060 to 1.0074 at 25°C/25°C.
- A hyperbaric solution may be prepared by mixing equal volumes of the 1% Solution and Dextrose Solution 10% (which is available in ampuls of 3 mL).
- If the NIPHANOID form is preferred, it is first dissolved in Dextrose Solution 10% in a ratio of 1 mL dextrose to 10 mg of the anesthetic. Further dilution is made with an equal volume of spinal fluid. The resulting solution now contains 5% dextrose with 5 mg of anesthetic agent per milliliter.
- A hypobaric solution may be prepared by dissolving the NIPHANOID in Sterile Water for Injection, USP (1 mg per milliliter). The specific gravity of this solution is essentially the same as that of water, 1.000 at 25°C/25°C.
- Examine ampuls carefully before use. Do not use solution if crystals, cloudiness, or discoloration is observed.
- These formulations of tetracaine hydrochloride do not contain preservatives; therefore, unused portions should be discarded and the reconstituted NIPHANOID should be used immediately.
STERILIZATION OF AMPULS
- The drug in intact ampuls is sterile. The preferred method of destroying bacteria on the exterior of ampuls before opening is heat sterilization (autoclaving). Immersion in antiseptic solution is not recommended.
- Autoclave at 15-pound pressure, at 121°C (250°F), for 15 minutes. The NIPHANOID form may also be autoclaved in the same way but may lose its snowlike appearance and tend to adhere to the sides of the ampul. This may slightly decrease the rate at which the drug dissolves but does not interfere with its anesthetic potency.
- Autoclaving increases likelihood of crystal formation. Unused autoclaved ampuls should be discarded. Under no circumstance should unused ampuls which have been autoclaved be returned to stock.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Tetracaine (injection) in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Tetracaine (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of tetracaine in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Tetracaine (injection) in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Tetracaine (injection) in pediatric patients.
Contraindications
- Spinal anesthesia with tetracaine is contraindicated in patients with known hypersensitivity to tetracaine hydrochloride or to drugs of a similar chemical configuration (ester-type local anesthetics), or aminobenzoic acid or its derivatives; and in patients for whom spinal anesthesia as a technique is contraindicated.
- The decision as to whether or not spinal anesthesia should be used for an individual patient should be made by the physician after weighing the advantages with the risks and possible complications. Contraindications to spinal anesthesia as a technique can be found in standard reference texts, and usually include generalized septicemia, infection at the site of injection, certain diseases of the cerebrospinal system, uncontrolled hypotension, etc.
Warnings
- RESUSCITATIVE EQUIPMENT AND DRUGS SHOULD BE IMMEDIATELY AVAILABLE WHENEVER ANY LOCAL ANESTHETIC DRUG IS USED.
- Large doses of local anesthetics should not be used in patients with heartblock.
- Reactions resulting in fatality have occurred on rare occasions with the use of local anesthetics, even in the absence of a history of hypersensitivity.
- Contains acetone sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Precautions
- The safety and effectiveness of any spinal anesthetic depend upon proper dosage, correct technique, adequate precautions, and readiness for emergencies. The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious systemic side effects. Tolerance varies with the status of the patient; debilitated, elderly patients or acutely ill patients should be given reduced doses commensurate with their weight, age, and physical status. Reduced doses are also indicated for obstetric patients and those with increased intra-abdominal pressure.
- Caution should be used in administering tetracaine to patients with abnormal or reduced levels of plasma esterases.
- Blood pressure should be frequently monitored during spinal anesthesia and hypotension immediately corrected.
- Spinal anesthetics should be used with caution in patients with severe disturbances of cardiac rhythm, shock, or heartblock.
Adverse Reactions
Clinical Trials Experience
- Systemic adverse reactions to tetracaine are characteristic of those associated with other local anesthetics and can involve the central nervous system and the cardiovascular system. Systemic reactions usually result from high plasma levels due to excessive dosage, rapid absorption, or inadvertent intravascular injection.
- A small number of reactions to tetracaine may result from hypersensitivity, idiosyncrasy or diminished tolerance to normal dosage.
- Central nervous system effects are characterized by excitation or depression. The first manifestation may be nervousness, dizziness, blurred vision, or tremors, followed by drowsiness, convulsions, unconsciousness and possibly respiratory and cardiac arrest. Since excitement may be transient or absent, the first manifestation may be drowsiness, sometimes merging into unconsciousness and respiratory and cardiac arrest. Other central nervous system effects may be nausea, vomiting, chills, constriction of the pupils, or tinnitus.
- Cardiovascular system reactions include depression of the myocardium, blood pressure changes (usually hypotension), and cardiac arrest.
- Allergic reactions, which may be due to hypersensitivity, idiosyncrasy, or diminished tolerance, are characterized by cutaneous lesions (eg, urticaria), edema, and other manifestations of allergy. Detection of sensitivity by skin testing is of limited value. Severe allergic reactions including anaphylaxis have occurred rarely and are not usually dose-related.
Reactions Associated with Spinal Anesthesia Techniques:
- Central Nervous System: post-spinal headache, meningismus, arachnoiditis, palsies, or spinal nerve paralysis.
- Cardiovascular: hypotension due to vasomotor paralysis and pooling of the blood in the venous bed.
- Respiratory: respiratory impairment or paralysis due to the level of anesthesia extending to the upper thoracic and cervical segments.
- Treatment of Reactions: Toxic effects of local anesthetics require symptomatic treatment; there is no specific cure.The most important measure is oxygenation of the patient by maintaining an airway and supporting ventilation. Supportive treatment of the cardiovascular system includes intravenous fluids and, when appropriate, vasopressors (preferably those that stimulate the myocardium). Convulsions are usually controlled with adequate oxygenation alone but intravenous administration, in small increments of a barbiturate (preferably an ultrashort-acting barbiturate such as thiopental and thiamylal), or diazepam can be utilized. Intravenous barbiturates or anticonvulsant agents should only be administered by those familiar with their use and only if ventilation and oxygenation have first been assured. In spinal anesthesia, sympathetic blockade also occurs as a pharmacological action, resulting in peripheral vasodilation and often hypotension. The extent of the hypotension will usually depend on the number of dermatomes blocked. The blood pressure should therefore be monitored in the early phases of anesthesia. If hypotension occurs, it is readily controlled by vasoconstrictors administered either by the intramuscular or the intravenous route, the dosage of which would depend on the severity of the hypotension and the response to treatment.
Postmarketing Experience
- There is limited information regarding postmarketing experience.
Drug Interactions
- Tetracaine should not be used if the patient is being treated with a sulfonamide because aminobenzoic acid inhibits the action of sulfonamides.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with tetracaine. It is not known whether tetracaine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Tetracaine should be given to a pregnant woman only if clearly needed and the potential benefits outweigh the risk.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tetracaine (injection) in women who are pregnant.
Labor and Delivery
- Vasopressor agents administered for the treatment of hypotension resulting from spinal anesthesia may result in severe persistent hypertension and/or rupture of cerebral blood vessels if oxytocic drugs have also been administered; therefore, vasopressors should be used with extreme caution in the presence of oxytocic drugs.
- Tetracaine has a recognized use during labor and delivery; the effect of the drug on duration of labor, incidence of forceps delivery, status of the newborn, and later growth and development of the child have not been studied.
Nursing Mothers
- It is not known whether tetracaine is excreted in human milk; however, it is rapidly metabolized following absorption into the plasma. Because many drugs are excreted in human milk, caution should be exercised when tetracaine is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness of tetracaine in pediatric patients have not been established.
Geriatic Use
- Clinical studies of tetracaine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Tetracaine (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tetracaine (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tetracaine (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tetracaine (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tetracaine (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tetracaine (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Subarachnoid.
Monitoring
- Blood pressure should be frequently monitored during spinal anesthesia and hypotension immediately corrected.
IV Compatibility
- There is limited information regarding IV Compatibility.
Overdosage
- There is limited information regarding Overdose.
Pharmacology
| |
Tetracaine (injection)
| |
| Systematic (IUPAC) name | |
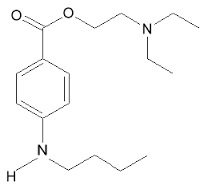
| 2-(dimethylamino)ethyl 4-(butylamino)benzoate | |
| Identifiers | |
| CAS number | 136-47-0 (hydrochloride) |
| ATC code | C05 D04AB06 (WHO) N01BA03 (WHO) S01HA03 (WHO) |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 264.363 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 75.6 |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Rx Only |
| Routes | Topical, Epidural, Spinal |
Mechanism of Action
- Parenteral administration of tetracaine stabilizes the neuronal membrane and prevents initiation and transmission of nerve impulses thereby effecting local anesthesia.
- The onset of action is rapid, and the duration prolonged (up to two or three hours or longer of surgical anesthesia).
- Tetracaine is detoxified by plasma esterases to aminobenzoic acid and diethylaminoethanol.
Structure
- Tetracaine hydrochloride is 2-(Dimethylamino)ethyl p-(butylamino)benzoate monohydrochloride. It is a white crystalline, odorless powder that is readily soluble in water, physiologic saline solution, and dextrose solution. It has the following structural formula:

- Tetracaine hydrochloride is a local anesthetic of the ester-linkage type, related to procaine.
- PONTOCAINE® hydrochloride is supplied in two forms for prolonged spinal anesthesia: Niphanoid® and 1% Solution.
- NIPHANOID: A sterile, instantly soluble form consisting of a network of extremely fine, highly purified particles, resembling snow.
- 1% Solution: A sterile, isotonic, isobaric solution, each 1 mL containing 10 mg tetracaine hydrochloride, 6.7 mg sodium chloride, and not more than 2 mg acetone sodium bisulfite. The air in the ampuls has been displaced by nitrogen gas or carbon dioxide gas. The pH is 3.2 to 6.
- Tetracaine hydrochloride is supplied for prolonged spinal anesthesia as: 1% Tetracaine Hydrochloride Injection, USP: A sterile, isotonic, isobaric solution, each 1 mL containing 10 mg tetracaine hydrochloride, 6.7 mg sodium chloride, and not more than 2 mg acetone sodium bisulfite. The air in the ampuls has been displaced by nitrogen gas or carbon dioxide gas. The pH is 3.2 to 6.
- These formulations do not contain preservatives.
Pharmacodynamics
- There is limited information regarding pharmacodynamics.
Pharmacokinetics
- There is limited information regarding pharmacokinetics.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term animal studies to evaluate carcinogenic potential and reproduction studies in animals have not been performed. There is no evidence from human data that tetracaine may be carcinogenic or that it impairs fertility.
Clinical Studies
- There is limited information regarding Clinical Studies.
How Supplied
- Tetracaine, 1% Solution:
- Uni-Amp® unit dose, NDC 0409-1846-02, pack of 20 mg/2 mL (10 mg/mL), box of 25.
Ampuls of 20 mg/2 mL (10 mg/mL), NDC 0409-1846-02, box of 25.
- Tetracaine Hydrochloride Injection, USP, 1% Solution:
- Bulk Pack ― 20 mg/2 mL (10 mg/mL), NDC 0409-1846-12, case of 400.
Protect ampuls from light. Store the 1% Solution under refrigeration 2 to 8ºC (36 to 46ºF).
- NIPHANOID® (instantly soluble powder):
- Ampuls of 20 mg, box of 100, NDC 0409-1849-06.
Storage
- Protect ampuls from light. Store the 1% Solution under refrigeration 2 to 8ºC (36 to 46ºF).
- Tetracaine Hydrochloride Injection supplied as a component of spinal anesthesia trays may be stored at 20 to 25°C (68 to 77°F) [see USP Controlled Room Temperature] for 12 months.
- Protect ampuls from light. Store solution at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Images
Drug Images
{{#ask: Page Name::Tetracaine (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Tetracaine (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- There is limited information regarding Clinical Studies.
Precautions with Alcohol
Alcohol-Tetracaine (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- NIPHANOID®[1]
Look-Alike Drug Names
- There is limited information regarding Look-Alike Drug Names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.