Tenofovir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS
See full prescribing information for complete Boxed Warning.
|
Overview
Tenofovir is a HIV-1 reverse transcriptase inhibitor and an HBV reverse transcriptase inhibitor that is FDA approved for the {{{indicationType}}} of HIV-1 infection in adults and pediatric patients 2 years of age and older, chronic hepatitis B in adults and pediatric patients 12 years of age and older. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, diarrhea, headache, pain, depression, asthenia, and nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
HIV-1 Infection
- Dosing Information
- The dose is one 300 mg VIREAD tablet once daily taken orally, without regard to food.
Chronic Hepatitis B
- Dosing Information
- The dose is one 300 mg VIREAD tablet once daily taken orally, without regard to food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
HIV infection - Type B viral hepatitis, chronic
- Developed by: The Department of Health and Human Services Panel on Antiretroviral Guidelines and the American Association for the Study of Liver Diseases (AASLD)
- Class of Recommendation: Adult, Class IIa
- Strength of Evidence: Adult, Category B
- Dosing Information
- Tenofovir 300 mg/day[1]
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tenofovir in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
HIV-1 Infection
- Pediatric Patients 12 Years of Age and Older (35 kg or more)
- The dose is one 300 mg VIREAD tablet once daily taken orally, without regard to food.
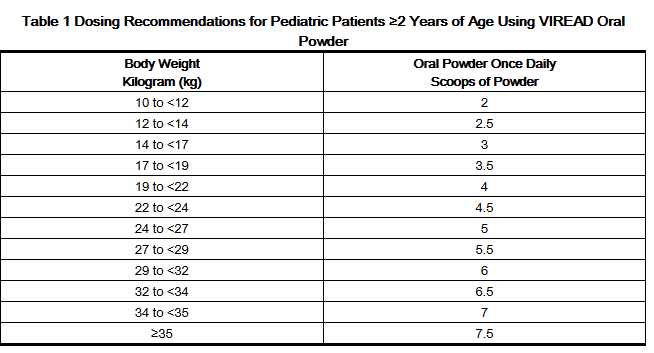
- For the treatment of HIV-1 in pediatric patients 2 years of age and older, the recommended oral dose of VIREAD is 8 mg of tenofovir disoproxil fumarate per kilogram of body weight (up to a maximum of 300 mg) once daily administered as oral powder or tablets.
- VIREAD oral powder should be measured only with the supplied dosing scoop. One level scoop delivers 1 g of powder which contains 40 mg of tenofovir disoproxil fumarate. VIREAD oral powder should be mixed in a container with 2 to 4 ounces of soft food not requiring chewing (e.g., applesauce, baby food, yogurt). The entire mixture should be ingested immediately to avoid a bitter taste. Do not administer VIREAD oral powder in a liquid as the powder may float on top of the liquid even after stirring. Further patient instructions on how to administer VIREAD oral powder with the supplied dosing scoop are provided in the FDA-approved patient labeling (Patient Information).
- VIREAD is also available as tablets in 150, 200, 250 and 300 mg strengths for pediatric patients who weigh greater than or equal to 17 kg and who are able to reliably swallow intact tablets. The dose is one tablet once daily taken orally, without regard to food.
- Tables 1 and 2 contain dosing recommendations for VIREAD oral powder and tablets based on body weight. Weight should be monitored periodically and the VIREAD dose adjusted accordingly.
Chronic Hepatitis B
- Pediatric Patients 12 Years of Age and Older (35 kg or more)
- The dose is one 300 mg VIREAD tablet once daily taken orally, without regard to food.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tenofovir in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tenofovir in pediatric patients.
Contraindications
- None
Warnings
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS
See full prescribing information for complete Boxed Warning.
|
Precautions
- Lactic Acidosis/Severe Hepatomegaly with Steatosis
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, in combination with other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogs to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with VIREAD should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
- Exacerbation of Hepatitis after Discontinuation of Treatment
- Discontinuation of anti-HBV therapy, including VIREAD, may be associated with severe acute exacerbations of hepatitis. Patients infected with HBV who discontinue VIREAD should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, resumption of anti-hepatitis B therapy may be warranted.
- New Onset or Worsening Renal Impairment
- Tenofovir is principally eliminated by the kidney. Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of VIREAD [See Adverse Reactions (6.2)].
- It is recommended that estimated creatinine clearance be assessed in all patients prior to initiating therapy and as clinically appropriate during therapy with VIREAD. In patients at risk of renal dysfunction, including patients who have previously experienced renal events while receiving HEPSERA®, it is recommended that estimated creatinine clearance, serum phosphorus, urine glucose, and urine protein be assessed prior to initiation of VIREAD, and periodically during VIREAD therapy.
- Dosing interval adjustment of VIREAD and close monitoring of renal function are recommended in all patients with creatinine clearance below 50 mL/min [See Dosage and Administration (2.3)]. No safety or efficacy data are available in patients with renal impairment who received VIREAD using these dosing guidelines, so the potential benefit of VIREAD therapy should be assessed against the potential risk of renal toxicity.
- VIREAD should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple non-steroidal anti-inflammatory drugs (NSAIDs)) [See Drug Interactions (7.3)]. Cases of acute renal failure after initiation of high dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on tenofovir DF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
- Persistent or worsening bone pain, pain in extremities, fractures and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in at-risk patients.
- Coadministration with Other Products
- VIREAD should not be used in combination with the fixed-dose combination products ATRIPLA, COMPLERA, STRIBILD, or TRUVADA since tenofovir disoproxil fumarate is a component of these products.
- VIREAD should not be administered in combination with HEPSERA (adefovir dipivoxil) [See Drug Interactions (7.3)].
- Patients Coinfected with HIV-1 and HBV
- Due to the risk of development of HIV-1 resistance, VIREAD should only be used in HIV-1 and HBV coinfected patients as part of an appropriate antiretroviral combination regimen.
- HIV-1 antibody testing should be offered to all HBV-infected patients before initiating therapy with VIREAD. It is also recommended that all patients with HIV-1 be tested for the presence of chronic hepatitis B before initiating treatment with VIREAD.
- Bone Effects
- Bone Mineral Density:
- In clinical trials in HIV-1 infected adults, VIREAD was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism, suggesting increased bone turnover relative to comparators. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher in subjects receiving VIREAD [See Adverse Reactions (6.1)].
- Clinical trials evaluating VIREAD in pediatric and adolescent subjects were conducted. Under normal circumstances, BMD increases rapidly in pediatric patients. In HIV-1 infected subjects aged 2 years to less than 18 years, bone effects were similar to those observed in adult subjects and suggest increased bone turnover. Total body BMD gain was less in the VIREAD-treated HIV-1 infected pediatric subjects as compared to the control groups. Similar trends were observed in chronic hepatitis B infected adolescent subjects aged 12 years to less than 18 years. In all pediatric trials, skeletal growth (height) appeared to be unaffected. [See Adverse Reactions (6.1)].
- The effects of VIREAD-associated changes in BMD and biochemical markers on long-term bone health and future fracture risk are unknown. Assessment of BMD should be considered for adults and pediatric patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial for all patients. If bone abnormalities are suspected then appropriate consultation should be obtained.
- Bone Mineral Density:
- Mineralization Defects:
- Cases of osteomalacia associated with proximal renal tubulopathy, manifested as bone pain or pain in extremities and which may contribute to fractures, have been reported in association with the use of VIREAD [See Adverse Reactions (6.2)]. Arthralgias and muscle pain or weakness have also been reported in cases of proximal renal tubulopathy. Hypophosphatemia and osteomalacia secondary to proximal renal tubulopathy should be considered in patients at risk of renal dysfunction who present with persistent or worsening bone or muscle symptoms while receiving products containing tenofovir DF [See Warnings and Precautions (5.3)].
- Mineralization Defects:
- Fat Redistribution
- In HIV-infected patients redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving combination antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
- Immune Reconstitution Syndrome
- Immune reconstitution syndrome has been reported in HIV-infected patients treated with combination antiretroviral therapy, including VIREAD. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
- Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
- Early Virologic Failure
- Clinical trials in HIV-infected subjects have demonstrated that certain regimens that only contain three nucleoside reverse transcriptase inhibitors (NRTI) are generally less effective than triple drug regimens containing two NRTIs in combination with either a non-nucleoside reverse transcriptase inhibitor or a HIV-1 protease inhibitor. In particular, early virological failure and high rates of resistance substitutions have been reported. Triple nucleoside regimens should therefore be used with caution. Patients on a therapy utilizing a triple nucleoside-only regimen should be carefully monitored and considered for treatment modification.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials in Adult Patients with HIV-1 Infection
- More than 12,000 subjects have been treated with VIREAD alone or in combination with other antiretroviral medicinal products for periods of 28 days to 215 weeks in clinical trials and expanded access programs. A total of 1,544 subjects have received VIREAD 300 mg once daily in clinical trials; over 11,000 subjects have received VIREAD in expanded access programs.
- The most common adverse reactions (incidence greater than or equal to 10%, Grades 2–4) identified from any of the 3 large controlled clinical trials include rash, diarrhea, headache, pain, depression, asthenia, and nausea.
- Treatment-Naïve Patients
- Study 903 - Treatment-Emergent Adverse Reactions: The most common adverse reactions seen in a double-blind comparative controlled trial in which 600 treatment-naïve subjects received VIREAD (N=299) or stavudine (N=301) in combination with lamivudine and efavirenz for 144 weeks (Study 903) were mild to moderate gastrointestinal events and dizziness.
- Mild adverse reactions (Grade 1) were common with a similar incidence in both arms, and included dizziness, diarrhea, and nausea. Selected treatment-emergent moderate to severe adverse reactions are summarized in Table 4.
- Laboratory Abnormalities: With the exception of fasting cholesterol and fasting triglyceride elevations that were more common in the stavudine group (40% and 9%) compared with VIREAD (19% and 1%) respectively, laboratory abnormalities observed in this trial occurred with similar frequency in the VIREAD and stavudine treatment arms. A summary of Grades 3–4 laboratory abnormalities is provided in Table 5.
- Study 934 - Treatment Emergent Adverse Reactions: In Study 934, 511 antiretroviral-naïve subjects received either VIREAD + EMTRIVA® administered in combination with efavirenz (N=257) or zidovudine/lamivudine administered in combination with efavirenz (N=254). Adverse reactions observed in this trial were generally consistent with those seen in previous studies in treatment-experienced or treatment-naïve subjects (Table 6).
- Changes in Bone Mineral Density:
- In HIV-1 infected adult subjects in Study 903, there was a significantly greater mean percentage decrease from baseline in BMD at the lumbar spine in subjects receiving VIREAD + lamivudine + efavirenz (-2.2% ± 3.9) compared with subjects receiving stavudine + lamivudine + efavirenz (-1.0% ± 4.6) through 144 weeks. Changes in BMD at the hip were similar between the two treatment groups (-2.8% ± 3.5 in the VIREAD group vs. -2.4% ± 4.5 in the stavudine group). In both groups, the majority of the reduction in BMD occurred in the first 24–48 weeks of the trial and this reduction was sustained through Week 144. Twenty-eight percent of VIREAD-treated subjects vs. 21% of the stavudine-treated subjects lost at least 5% of BMD at the spine or 7% of BMD at the hip. Clinically relevant fractures (excluding fingers and toes) were reported in 4 subjects in the VIREAD group and 6 subjects in the stavudine group. In addition, there were significant increases in biochemical markers of bone metabolism (serum bone-specific alkaline phosphatase, serum osteocalcin, serum C telopeptide, and urinary N telopeptide) and higher serum parathyroid hormone levels and 1,25 Vitamin D levels in the VIREAD group relative to the stavudine group; however, except for bone-specific alkaline phosphatase, these changes resulted in values that remained within the normal range [See Warnings and Precautions (5.6)].
- Laboratory Abnormalities: Laboratory abnormalities observed in this trial were generally consistent with those seen in previous trials (Table 7).
- Treatment-Experienced Patients
- Treatment-Emergent Adverse Reactions: The adverse reactions seen in treatment experienced subjects were generally consistent with those seen in treatment naïve subjects including mild to moderate gastrointestinal events, such as nausea, diarrhea, vomiting, and flatulence. Less than 1% of subjects discontinued participation in the clinical trials due to gastrointestinal adverse reactions (Study 907).
- A summary of moderate to severe, treatment-emergent adverse reactions that occurred during the first 48 weeks of Study 907 is provided in Table 8.
- Laboratory Abnormalities: Laboratory abnormalities observed in this trial occurred with similar frequency in the VIREAD and placebo-treated groups. A summary of Grades 3–4 laboratory abnormalities is provided in Table 9.
Clinical Trials in Pediatric Subjects 2 Years of Age and Older with HIV-1 Infection
- Assessment of adverse reactions is based on two randomized trials (Studies 352 and 321) in 184 HIV-1 infected pediatric subjects (2 to less than 18 years of age) who received treatment with VIREAD (N=93) or placebo/active comparator (N=91) in combination with other antiretroviral agents for 48 weeks. The adverse reactions observed in subjects who received treatment with VIREAD were consistent with those observed in clinical trials in adults.
- Eighty-nine pediatric subjects (2 to less than 12 years of age) received VIREAD in Study 352 for a median exposure of 104 weeks. Of these, 4 subjects discontinued from the trial due to adverse reactions consistent with proximal renal tubulopathy. Three of these 4 subjects presented with hypophosphatemia and also had decreases in total body or spine BMD Z score [See Warnings and Precautions (5.6)].
- Changes in Bone Mineral Density:
- Clinical trials in HIV-1 infected children and adolescents evaluated BMD changes. In Study 321 (12 to less than 18 years), the mean rate of BMD gain at Week 48 was less in the VIREAD compared to the placebo treatment group. Six VIREAD treated subjects and one placebo treated subject had significant (greater than 4%) lumbar spine BMD loss at Week 48. Changes from baseline BMD Z-scores were –0.341 for lumbar spine and –0.458 for total body in the 28 subjects who were treated with VIREAD for 96 weeks. In Study 352 (2 to less than 12 years), the mean rate of BMD gain in lumbar spine at Week 48 was similar between the VIREAD and the d4T or AZT treatment groups. Total body BMD gain was less in the VIREAD compared to the d4T or AZT treatment groups. One VIREAD-treated subject and none of the d4T or AZT-treated subjects experienced significant (greater than 4%) lumbar spine BMD loss at Week 48. Changes from baseline in BMD Z scores were –0.012 for lumbar spine and –0.338 for total body in the 64 subjects who were treated with VIREAD for 96 weeks. In both trials, skeletal growth (height) appeared to be unaffected [See Warnings and Precautions (5.6)].
Clinical Trials in Adult Subjects with Chronic Hepatitis B and Compensated Liver Disease
- Treatment-Emergent Adverse Reactions: In controlled clinical trials in 641 subjects with chronic hepatitis B (0102 and 0103), more subjects treated with VIREAD during the 48-week double-blind period experienced nausea: 9% with VIREAD versus 2% with HEPSERA. Other treatment-emergent adverse reactions reported in more than 5% of subjects treated with VIREAD included: abdominal pain, diarrhea, headache, dizziness, fatigue, nasopharyngitis, back pain and skin rash.
- During the open-label phase of treatment with VIREAD (weeks 48–240) in Studies 0102 and 0103, less than 1% of subjects (5/585) experienced a confirmed increase in serum creatinine of 0.5 mg/dL from baseline. No significant change in the tolerability profile was observed with continued treatment for up to 240 weeks.
- Laboratory Abnormalities: A summary of Grades 3–4 laboratory abnormalities through Week 48 is provided in Table 10. Grades 3–4 laboratory abnormalities were similar in subjects continuing VIREAD treatment for up to 240 weeks in these trials.
- The overall incidence of on-treatment ALT flares (defined as serum ALT greater than 2 × baseline and greater than 10 × ULN, with or without associated symptoms) was similar between VIREAD (2.6%) and HEPSERA (2%). ALT flares generally occurred within the first 4–8 weeks of treatment and were accompanied by decreases in HBV DNA levels. No subject had evidence of decompensation. ALT flares typically resolved within 4 to 8 weeks without changes in study medication.
- The adverse reactions observed in subjects with chronic hepatitis B and lamivudine resistance who received treatment with VIREAD were consistent with those observed in other hepatitis B clinical trials in adults.
Clinical Trials in Adult Subjects with Chronic Hepatitis B and Decompensated Liver Disease
- In a small randomized, double-blind, active-controlled trial (0108), subjects with CHB and decompensated liver disease received treatment with VIREAD or other antiviral drugs for up to 48 weeks [See Clinical Studies (14.2)]. Among the 45 subjects receiving VIREAD, the most frequently reported treatment-emergent adverse reactions of any severity were abdominal pain (22%), nausea (20%), insomnia (18%), pruritus (16%), vomiting (13%), dizziness (13%), and pyrexia (11%). Two of 45 (4%) subjects died through Week 48 of the trial due to progression of liver disease. Three of 45 (7%) subjects discontinued treatment due to an adverse event. Four of 45 (9%) subjects experienced a confirmed increase in serum creatinine of 0.5 mg/dL (1 subject also had a confirmed serum phosphorus less than 2 mg/dL through Week 48). Three of these subjects (each of whom had a Child-Pugh score greater than or equal to 10 and MELD score greater than or equal to 14 at entry) developed renal failure. Because both VIREAD and decompensated liver disease may have an impact on renal function, the contribution of VIREAD to renal impairment in this population is difficult to ascertain.
- One of 45 subjects experienced an on-treatment hepatic flare during the 48 Week trial.
Clinical Trials in Pediatric Subjects 12 Years of Age and Older with Chronic Hepatitis B
- Assessment of adverse reactions is based on one randomized study (Study GS-US-174-0115) in 106 pediatric subjects (12 to less than 18 years of age) infected with chronic hepatitis B receiving treatment with VIREAD (N = 52) or placebo (N = 54) for 72 weeks. The adverse reactions observed in pediatric subjects who received treatment with VIREAD were consistent with those observed in clinical trials of VIREAD in adults.
- In this study, both the VIREAD and placebo treatment arms experienced an overall increase in mean lumbar spine BMD over 72 weeks, as expected for an adolescent population. The BMD gains from baseline to Week 72 in lumbar spine and total body BMD in VIREAD-treated subjects (+5% and +3%, respectively) were less than the BMD gains observed in placebo-treated subjects (+8% and +5%, respectively). Three subjects in the VIREAD group and two subjects in the placebo group had significant (greater than 4%) lumbar spine BMD loss at Week 72. At baseline, mean BMD Z-scores in subjects randomized to VIREAD were −0.43 for lumbar spine and −0.20 for total body, and mean BMD Z-scores in subjects randomized to placebo were −0.28 for lumbar spine and −0.26 for total body. In subjects receiving VIREAD for 72 weeks, the mean change in BMD Z-score was −0.05 for lumbar spine and −0.15 for total body compared to +0.07 and +0.06, respectively, in subjects receiving placebo. As observed in pediatric studies of HIV-infected patients, skeletal growth (height) appeared to be unaffected [See Warnings and Precautions (5.6)].
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of VIREAD. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tenofovir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tenofovir during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Tenofovir with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Tenofovir with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Tenofovir with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Tenofovir with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tenofovir with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tenofovir in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tenofovir in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tenofovir in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tenofovir in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Tenofovir in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Tenofovir in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Tenofovir in the drug label.
Pharmacology
There is limited information regarding Tenofovir Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Tenofovir in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Tenofovir in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Tenofovir in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Tenofovir in the drug label.
How Supplied
Storage
There is limited information regarding Tenofovir Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Tenofovir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tenofovir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Tenofovir in the drug label.
Precautions with Alcohol
- Alcohol-Tenofovir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[2]
Look-Alike Drug Names
- A® — B®[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Lacombe K, Gozlan J, Boyd A, Boelle PY, Bonnard P, Molina JM; et al. (2008). "Comparison of the antiviral activity of adefovir and tenofovir on hepatitis B virus in HIV-HBV-coinfected patients". Antivir Ther. 13 (5): 705–13. PMC 2665195. PMID 18771054.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Tenofovir |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Tenofovir |Label Name=Tenofovir11.png
}}
{{#subobject:
|Label Page=Tenofovir |Label Name=Tenofovir11.png
}}