Template:SN: Difference between revisions
| Line 74: | Line 74: | ||
|} | |} | ||
Patients with pernicious anemia usually have very low levels of acid in the stomach (achlorhydria) and high levels of fasting gastrin (hypergastrinemia). | |||
==Differentiating pernicious anemia from other diseases== | |||
Pernicious anemia shares many similarities with other forms of megaloblastic anemia like B12 and folate deficiency. | |||
*Vitamin B12 deficiency due to insufficient intake (eg veganism) has all the features of pernicious anemia like megaloblasts, hypersegmented neutrophils, neuropsychiatric manifestations. But atrophic gastritis is absent, so achlorhydria, parietal cell antibodies or IF antibodies are absent. Intrinsic factor levels are also normal. | |||
*Folic acid deficiency also results in megaloblastic anemia and similar hematological changes as pernicious anemia, but urinary excretion of methylmalonic acid is absent, so are features of pernicious anemia like achlorhydria, antibodies and normal IF levels. | |||
*Ileal resection causes B12 deficiency due to decreased absorption | |||
*Certain drugs such as methotrexate, azathioprine cause folate deficiency and result in megaloblastic anemia. This is usually seen in patients taking chemotherapy or other chronic conditions such as rheumatoid arthritis | |||
*Chronic proton pump inhibitor therapy also results in B12 deficiency as vitamin B12 cannot dissociate from its carrier protein in the absence of an acidic environment.<ref name="pmid25083257">{{cite journal| author=Heidelbaugh JJ| title=Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. | journal=Ther Adv Drug Saf | year= 2013 | volume= 4 | issue= 3 | pages= 125-33 | pmid=25083257 | doi=10.1177/2042098613482484 | pmc=4110863 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25083257 }} </ref> | |||
*Long term use of metformin, such as in diabetics, is linked to vitamin B12 deficiency and symptoms similar to pernicious anemia, but this can be differentiated from pernicious anemia as it is seen in diabetics on chronic therapy.<ref name="pmid26900641">{{cite journal| author=Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ | display-authors=etal| title=Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. | journal=J Clin Endocrinol Metab | year= 2016 | volume= 101 | issue= 4 | pages= 1754-61 | pmid=26900641 | doi=10.1210/jc.2015-3754 | pmc=4880159 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26900641 }} </ref> | |||
==Genetics== | ==Genetics== | ||
Revision as of 13:30, 25 June 2020
"sandbox:SN"
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief:
Overview
Pernicious anemia (also called Addison's anemia) is a type of red blood cell disorder caused by impaired vitamin B12 metabolism. Vitamin B12 is primarily absorbed by the small intestine, after being bound to intrinsic factor secreted by parietal cells of gastric mucosa. When this process is disrupted by conditions like atrophic gastritis, celiac disease, small bowel resection etc, B12 deficiency ensues. Historically, this type of anemia was called "pernicious" because it was harder to treat and most often resulted in death. Red blood cells in this type of anemia are abnormally large, and are called macrocytic.
Pathophysiology
Vitamin B12 is an essential vitamin for humans and animals because we cannot synthesise it on our own. B12 is a cofactor in DNA synthesis and other important biochemical reactions. Vitamin B12 deficiency manifests as anemia because hematopoetic stem cells in the bone marrow which are rapidly dividing need B12 for division and DNA production. This process is impaired leading to ineffective hematopoeisis. Vitamin B12 is also necessary for production of myelin which is an important component in the covering sheath of nerves. Deficiency results in improper nerve conduction due to nerve destabilisation.
Physiology
Vitamin B12 is also called cobalamin because it contains cobalt at the core of its structure. Dietary sources of vitamin B12 include meat, fish and eggs. When consumed through its dietary source, B12 is bound to protein till it enters the stomach. In the stomach, B12 is uncoupled from its carrier protein due to the presence of gastric acid, which is why vitamin B12 deficiency is so commonly seen among those on chronic antacid medication. Once in the stomach, it is then bound to gastric R binder, a glycoprotein secreted by the salivary glands till it reaches the duodenum. In the duodenum and jejunum, the pancreatic enzymes digest the gastric R binder and cobalamin is bound to intrinsic factor (IF). Intrinsic factor is secreted by the gastric parietal cells. Once bound to IF, vitamin B12 travels up to the ileum where IF is removed and B12 binds with carrier proteins called transcobalamins and this complex is taken up by the liver and bone marrow, among other tissues. Inside the cells, the transcobalamin-B12 complex is dissolved and cobalamin is reduced to methylcobalamin which serves as a cofactor and coenzyme in many important biochemical reactions[1]. The two major reactions involving B12 in the human body are:
- Vitamin B12 in the from of cyanocobalamin is required in the synthesis of methionine. Methionine is produced from homocysteine and is catalysed by the enzyme methionine synthase. This enzyme utilises cyanocobalamin as a cofactor. Deficiency of vitamin B12 causes a decreased production of methionine and buildup of homocysteine. Hyperhomocysteinemia is implicated as a risk factor in cardiovascular disease.[2]
- The Kreb's cycle utilises vitamin B12 in the reaction converting methylmalonyl-CoA to succinyl-CoA. Thus vitamin B12 deficiency causes a buildup of methylmalonic acid, the substrate for the enzyme methylmalonyl coenzyme A mutase. Methylmalonic acid levels are elevated in the urine of people affected with pernicious anemia and other forms of B12 deficiency.
Storage
The human body can store anywhere from 2-5mg of vitamin B12. Most of this is stored in the liver and is recycled via enterohepatic circulation.
Pathogenesis
Pernicious anemia is a type of megaloblastic anemia caused due to improper vitamin B12 absorption by the body. Impaired absorption occurs because of deficiency of intrinsic factor which is produced by the parietal cells of the stomach. The etiology of pernicious anemia can be due to autoimmune causes or genetic disease. In autoimmune disease, the antibodies attack most of the gastric mucosa, but the antrum is spared.
Autoimmune causes of pernicious anemia
This is the most common cause of pernicious anemia. In autoimmune pernicious anemia, the body produces antibodies against parietal cells or intrinsic factor.
- Antibodies against parietal cells of the gastric mucosa work to inhibit the H+/K(+)-ATPase which is the proton pump present in the parietal cells. The proton pump serves as an auto antigen and activates the cytotoxic CD4+ T cells which proceed to destroy gastric mucosal cells.[3][4]
- Intrinsic factor antibodies are present in fewer cases of pernicious anaemia but are highly specific. There are 2 types of IF antibodies. They prevent the binding and absorption of cobalamin in the ileum via its receptor.[5]
Clinical features
The symptoms of pernicious anemia take months, and often years to manifest. Patients most commonly present with symptoms of anemia like lightheadedness, dizziness, shortness of breath etc. The population affected with pernicious anemia is usually the elderly (>60 years) owing to its insidious onset. However, the genetic form presents in children below 15 years of age.
| Hematological symptoms | Gastrointestinal symptoms | Neurological symptoms |
|---|---|---|
| Pallor | Loss of appetite | Parasthesias |
| Icterus | Weight loss
|
Peripheral neuropathy |
| Shortness of breath | Nausea and vomiting | Ataxia |
| Dizziness | Smooth, beefy, red tongue - Glossitis | Weakness |
| Tachycardia | Diarrhea | Hyperreflexia |
| Fatigue | Confusion | |
| Depression |
Patients with pernicious anemia usually have very low levels of acid in the stomach (achlorhydria) and high levels of fasting gastrin (hypergastrinemia).
Differentiating pernicious anemia from other diseases
Pernicious anemia shares many similarities with other forms of megaloblastic anemia like B12 and folate deficiency.
- Vitamin B12 deficiency due to insufficient intake (eg veganism) has all the features of pernicious anemia like megaloblasts, hypersegmented neutrophils, neuropsychiatric manifestations. But atrophic gastritis is absent, so achlorhydria, parietal cell antibodies or IF antibodies are absent. Intrinsic factor levels are also normal.
- Folic acid deficiency also results in megaloblastic anemia and similar hematological changes as pernicious anemia, but urinary excretion of methylmalonic acid is absent, so are features of pernicious anemia like achlorhydria, antibodies and normal IF levels.
- Ileal resection causes B12 deficiency due to decreased absorption
- Certain drugs such as methotrexate, azathioprine cause folate deficiency and result in megaloblastic anemia. This is usually seen in patients taking chemotherapy or other chronic conditions such as rheumatoid arthritis
- Chronic proton pump inhibitor therapy also results in B12 deficiency as vitamin B12 cannot dissociate from its carrier protein in the absence of an acidic environment.[6]
- Long term use of metformin, such as in diabetics, is linked to vitamin B12 deficiency and symptoms similar to pernicious anemia, but this can be differentiated from pernicious anemia as it is seen in diabetics on chronic therapy.[7]
Genetics
Some forms of pernicious anemia are congenital and a genetic link has been postulated because of a higher incidence in certain populations. Affected people have a complete or near total absence of intrinsic factor and the presence of antibodies against intrinsic factor. The genetic variant is transmitted through an autosomal recessive pattern.[8]
Associated Conditions
People affected with pernicious anemia might have other coexisting autoimmune conditions such as autoimmune thyroiditis, autoimmune diabetes, vitiligo etc. Autoimmune thyroiditis is most commonly seen in patients with pernicious anemia, particularly females. HLA DR3 has been implicated in the development of autoimmune diseases such as pernicious anemia[9].
Signs and symptoms
The symptoms of pernicious anemia take months, and often years to manifest. Patients most commonly present with symptoms of anemia like lightheadedness, dizziness, shortness of breath etc. The population affected with pernicious anemia is usually the elderly (>60 years) owing to its insidious onset. However, the genetic form presents in children below 15 years of age.
| Hematological symptoms | Gastrointestinal symptoms | Neurological symptoms |
|---|---|---|
| Pallor | Loss of appetite | Parasthesias |
| Icterus | Weight loss
|
Peripheral neuropathy |
| Shortness of breath | Nausea and vomiting | Ataxia |
| Dizziness | Smooth, beefy, red tongue - Glossitis | Weakness |
| Tachycardia | Diarrhea | Hyperreflexia |
| Fatigue | Confusion | |
| Depression |
Physical examination findings
Most important physical examination findings are the neurological findings of long standing B12 deficiency which leads to subacute combined degeneration of the spinal cord.
- Hematological signs include pallor and icterus.
- Neurological signs: Vitamin B12 deficiency causes nerve demyelination. B12 deficiency also causes a buildup of methylmalonic acid which is toxic to neuronal cells and causes apoptosis.[10].
The main neurological manifestation of pernicious anemia and vitamin B12 deficiency is subacute combined degeneration. The posterior and lateral columns of the spinal cord are affected. Lateral column demyelination manifests as hyperreflexia and spasticity, while posterior column defects are loss of proprioception and vibration sense. Ataxia and loss of tandem gait are also manifestations of posterior column demyelination. Recreational or accidental inhalation of nitrous oxide gas (laughing gas) can precipitate subacute combined degeneration in people with low levels of vitamin B12.[11]
- Gastrointestinal signs: Upto 25% of people affected with pernicious anemia develop glossitis. The tongue appears red, "beefy" and smooth due to atrophy and blunting of the lingual papillae.[12]
Subacute combined degeneration

Microscopic Pathology
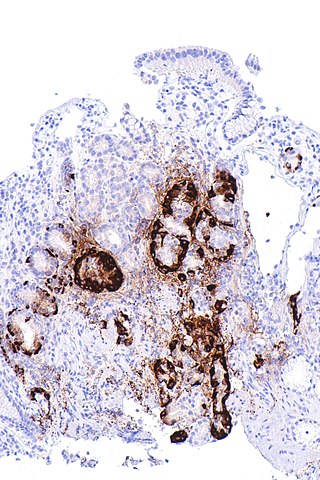
Atrophic gastritis in pernicious anemia is seen due to autoimmune (cytotoxic T cell) mediated destruction of the parietal cells of the gastric mucosa. The following changes can be seen on peripheral smear:
- The most obvious peripheral smear finding is megaloblasts and macrocytes.
Megaloblastic anemia results due to the lagging behind of nuclear development when compared to cytoplasmic development. This is known as nuclear-cytoplasmic asynchrony. Such defective cells are destroyed in the bone marrow (intramedullary hemolysis).
- Decreased number of RBCs (erythopenia)
- Macrocytosis- the RBCs in pernicious anemia are very large. Macrocytosis is defined as cells that have an MCV >100 femtolitres (normal :80-100fL)
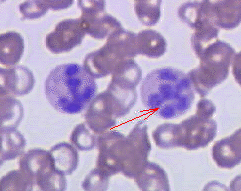
- Hypersegmented neutrophils : Neutrophils containing ≥ 6 lobes.
- Poikilocytosis and anisocytosis
- Low reticulocyte count (reticulopenia)
- Howell-Jolly bodies
-
Atrophic gastritis
-
Hypersegmented neutrophil
Diagnosis
- The first step in diagnosis is a blood vitamin B12 level. Blood levels less than 200 pg/ml are seen in pernicious anemia.
- Once a low level is confirmed, further tests like CBC, folate level, peripheral smear are done.
- The most crucial step in diagnosing pernicious anemia is testing for intrinsic factor antibodies and parietal cell antibodies.
- Intrinsic factor testing reveals a low level.[13]
- Gastric mucosal sampling shows parietal cell atrophy with antral sparing.
- Increased level of gastrin.
- Increased levels of homocysteine and methylmalonyl-CoA.
- Decreased folate levels are seen due to "folate trapping" in the form of methyltetrahydrofolate.
Pernicious anemia was historically diagnosed with a Shilling Test.
Shilling Test
The Shilling test is no longer done to detect an IF deficiency but has historical importance. After a vitamin B12 deficiency is noted, the patient is given radioactively tagged cobalamin to take orally. Soon after this step, the patient is injected with unlabelled cobalamin intramuscularly. Urine is checked for radioactive cobalamin for the next 24 hours. In pernicious anemia, there is an intrinsic factor deficiency, therefore the orally consumed radioactive cobalamin will not be absorbed and can be detected in the urine. In the next step, the patient is given radioactive cobalamin along with intrinsic factor and their urine is checked for traces of radioactive cobalamin. Absence of radioactive cobalamin in the urine points to the deficiency of intrinsic factor in the patients stomach which is the cause of vitamin B12 deficiency[14]. If the cobalamin absorption does not increase even with intrinsic factor supplementation, patient can be given a course of antibiotics as bacterial overgrowth may hinder absorption.
References
- ↑ Harrington DJ (2017). "Laboratory assessment of vitamin B12 status". J Clin Pathol. 70 (2): 168–173. doi:10.1136/jclinpath-2015-203502. PMID 27169753.
- ↑ Tinelli C, Di Pino A, Ficulle E, Marcelli S, Feligioni M (2019). "Hyperhomocysteinemia as a Risk Factor and Potential Nutraceutical Target for Certain Pathologies". Front Nutr. 6: 49. doi:10.3389/fnut.2019.00049. PMC 6491750. PMID 31069230.
- ↑ Callaghan JM, Khan MA, Alderuccio F, van Driel IR, Gleeson PA, Toh BH (1993). "Alpha and beta subunits of the gastric H+/K(+)-ATPase are concordantly targeted by parietal cell autoantibodies associated with autoimmune gastritis". Autoimmunity. 16 (4): 289–95. doi:10.3109/08916939309014648. PMID 7517707.
- ↑ Toh BH, Sentry JW, Alderuccio F (2000). "The causative H+/K+ ATPase antigen in the pathogenesis of autoimmune gastritis". Immunol Today. 21 (7): 348–54. doi:10.1016/s0167-5699(00)01653-4. PMID 10871877.
- ↑ Schade SG, Abels J, Schilling RF (1967). "Studies on antibody to intrinsic factor". J Clin Invest. 46 (4): 615–20. doi:10.1172/JCI105563. PMC 442045. PMID 6021209.
- ↑ Heidelbaugh JJ (2013). "Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications". Ther Adv Drug Saf. 4 (3): 125–33. doi:10.1177/2042098613482484. PMC 4110863. PMID 25083257.
- ↑ Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ; et al. (2016). "Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study". J Clin Endocrinol Metab. 101 (4): 1754–61. doi:10.1210/jc.2015-3754. PMC 4880159. PMID 26900641.
- ↑ Gordon MM, Brada N, Remacha A, Badell I, del Río E, Baiget M; et al. (2004). "A genetic polymorphism in the coding region of the gastric intrinsic factor gene (GIF) is associated with congenital intrinsic factor deficiency". Hum Mutat. 23 (1): 85–91. doi:10.1002/humu.10297. PMID 14695536.
- ↑ Zulfiqar AA, Andres E (2017). "Association pernicious anemia and autoimmune polyendocrinopathy: a retrospective study". J Med Life. 10 (4): 250–253. PMC 5771255. PMID 29362601.
- ↑ Han L, Wu S, Han F, Gu X (2015). "Insights into the molecular mechanisms of methylmalonic acidemia using microarray technology". Int J Clin Exp Med. 8 (6): 8866–79. PMC 4538064. PMID https://www.ncbi.nlm.nih.gov/pubmed/26309541 Check
|pmid=value (help). - ↑ Choi C, Kim T, Park KD, Lim OK, Lee JK (2019). "Subacute Combined Degeneration Caused by Nitrous Oxide Intoxication: A Report of Two Cases". Ann Rehabil Med. 43 (4): 530–534. doi:10.5535/arm.2019.43.4.530. PMC 6734019 Check
|pmc=value (help). PMID 31499607. - ↑ Stoopler ET, Kuperstein AS (2013). "Glossitis secondary to vitamin B12 deficiency anemia". CMAJ. 185 (12): E582. doi:10.1503/cmaj.120970. PMC 3761039. PMID 23359038.
- ↑ Lahner E, Annibale B (2009). "Pernicious anemia: new insights from a gastroenterological point of view". World J Gastroenterol. 15 (41): 5121–8. doi:10.3748/wjg.15.5121. PMC 2773890. PMID 19891010.
- ↑ "StatPearls". 2020. PMID 29939561.