Telavancin hydrochloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warnings
See full prescribing information for complete Boxed Warning.
* Patients with pre-existing moderate/severe renal impairment (CrCl ≤ 50 mL/min) who were treated with VIBATIV for hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia had increased mortality observed versus vancomycin. Use of VIBATIV in patients with pre-existing moderate/severe renal impairment (CrCl≤ 50 mL/min) should be considered only when the anticipated benefit to the patient outweighs the potential risk.
|
Overview
Telavancin hydrochloride is an antibiotic that is FDA approved for the treatment of Complicated skin and skin structure infections (cSSSI) and Hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP). There is a Black Box Warning for this drug as shown here. Common adverse reactions include taste disturbance, nausea, vomiting, and foamy urine.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Complicated Skin and Skin Structure Infections
- Caused by susceptible isolates of the following Gram-positive microorganisms: Staphylococcus aureus (including methicillin-susceptible and -resistant isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus), or Enterococcus faecalis (vancomycin-susceptible isolates only).
- Dosage: 10 mg/kg administered over a 60-minute period by intravenous infusion once every 24 hours for 7 to 14 days.
HABP/VABP
- Caused by susceptible isolates of Staphylococcus aureus (including methicillin-susceptible and -resistant isolates). VIBATIV should be reserved for use when alternative treatments are not suitable.
- Dosage: 10 mg/kg administered over a 60-minute period by intravenous infusion once every 24 hours for 7 to 21 days.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Telavancin hydrochloride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Telavancin hydrochloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness not established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Telavancin hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Telavancin hydrochloride in pediatric patients.
Contraindications
- Intravenous Unfractionated Heparin Sodium because the activated partial thromboplastin time (aPTT) test results are expected to be artificially prolonged for 0 to 18 hours after VIBATIV administration.
- Known Hypersensitivity to VIBATIV
Warnings
|
Warnings
See full prescribing information for complete Boxed Warning.
* Patients with pre-existing moderate/severe renal impairment (CrCl ≤ 50 mL/min) who were treated with VIBATIV for hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia had increased mortality observed versus vancomycin. Use of VIBATIV in patients with pre-existing moderate/severe renal impairment (CrCl≤ 50 mL/min) should be considered only when the anticipated benefit to the patient outweighs the potential risk.
|
There is limited information regarding Telavancin hydrochloride Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Telavancin hydrochloride Clinical Trials Experience in the drug label.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of VIBATIV. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serious hypersensitivity reactions have been reported after first or subsequent doses of VIBATIV, including anaphylactic reactions. It is unknown if patients with hypersensitivity reactions to vancomycin will experience cross-reactivity to telavancin.
Drug Interactions
Effects of Telavancin on Coagulation Test Parameters
Telavancin binds to the artificial phospholipid surfaces added to common anticoagulation tests, thereby interfering with the ability of the coagulation complexes to assemble on the surface of the phospholipids and promote clotting in vitro. These effects appear to depend on the type of reagents used in commercially available assays. Thus, when measured shortly after completion of an infusion of VIBATIV, increases in the PT, INR, aPTT, and ACT have been observed. These effects dissipate over time, as plasma concentrations of telavancin decrease.
Urine Protein Tests
Telavancin interferes with urine qualitative dipstick protein assays, as well as quantitative dye methods (e.g., pyrogallol red-molybdate). However, microalbumin assays are not affected and can be used to monitor urinary protein excretion during VIBATIV treatment.
Use in Specific Populations
Pregnancy
Fetal Risk Summary
All pregnancies have a background risk of birth defects (about 3%), pregnancy loss (about 15%), or other adverse outcomes regardless of drug exposure.
There are no data on VIBATIV use in pregnant women. In 3 animal species, VIBATIV exposure during pregnancy at clinically relevant doses caused reduced fetal weights and increased rates of digit and limb malformations in offspring. These data raise concern about potential adverse developmental outcomes in humans (see DATA).
Clinical Considerations
Given the lack of human data and the risks suggested by animal data, avoid using VIBATIV in pregnant women unless the benefits to the patient outweigh the potential risks to the fetus.
Human Data
There are no data on human pregnancies exposed to VIBATIV.
Animal Data
In embryo-fetal development studies in rats, rabbits, and minipigs, telavancin demonstrated the potential to cause limb and skeletal malformations when given intravenously during the period of organogenesis at doses up to 150, 45, or 75 mg/kg/day, respectively. These doses resulted in exposure levels approximately 1- to 2-fold the human exposure (AUC) at the maximum clinical recommended dose. Malformations observed at <1% (but absent or at lower rates in historical or concurrent controls), included brachymelia (rats and rabbits), syndactyly (rats, minipigs), adactyly (rabbits), and polydactyly (minipigs). Additional findings in rabbits included flexed front paw and absent ulna, and in the minipigs included misshapen digits and deformed front leg. Fetal body weights were decreased in rats.
In a prenatal/perinatal development study, pregnant rats received intravenous telavancin at up to 150 mg/kg/day (approximately the same AUC as observed at the maximum clinical dose) from the start of organogenesis through lactation. Offspring showed decreases in fetal body weight and an increase in the number of stillborn pups. Brachymelia was also observed. Developmental milestones and fertility of the pups were unaffected.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Telavancin hydrochloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Telavancin hydrochloride during labor and delivery.
Nursing Mothers
It is not known whether telavancin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when VIBATIV is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of VIBATIV in pediatric patients has not been studied.
Geriatic Use
Of the 929 patients treated with VIBATIV at a dose of 10 mg/kg once daily in clinical trials of cSSSI, 174 (19%) were ≥65 years of age and 87 (9%) were ≥75 years of age. In the cSSSI trials, lower clinical cure rates were observed in patients ≥65 years of age compared with those <65 years of age. Overall, treatment-emergent adverse events occurred with similar frequencies in patients ≥65 (75% of patients) and <65 years of age (83% of patients). Fifteen of 174 (9%) patients ≥65 years of age treated with VIBATIV had adverse events indicative of renal impairment compared with 16 of 755 (2%) patients <65 years of age.
Of the 749 HABP/VABP patients treated with VIBATIV at a dose of 10 mg/kg once daily in clinical trials of HABP/VABP, 397 (53%) were ≥65 years of age and 230 (31%) were ≥75 years of age. Treatment-emergent adverse events as well as deaths and other serious adverse events occurred more often in patients ≥65 years of age than in those <65 years of age in both treatment groups.
Telavancin is substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection in this age group.
The mean plasma AUC values of telavancin were similar in healthy young and elderly subjects. Dosage adjustment for elderly patients should be based on renal function.
Gender
The impact of gender on the pharmacokinetics of telavancin was evaluated in healthy male (n=8) and female (n=8) subjects. The pharmacokinetics of telavancin were similar in males and females. No dosage adjustment is recommended based on gender.
Race
There is no FDA guidance on the use of Telavancin hydrochloride with respect to specific racial populations.
Renal Impairment
The HABP/VABP and cSSSI trials included patients with normal renal function and patients with varying degrees of renal impairment. Patients with underlying renal dysfunction or risk factors for renal dysfunction had a higher incidence of renal adverse events.
In the HABP/VABP studies higher mortality rates were observed in the VIBATIV-treated patients with baseline CrCl≤50 mL/min. Use of VIBATIV in patients with pre-existing moderate/severe renal impairment should be considered only when the anticipated benefit to the patient outweighs the potential risk.
VIBATIV-treated patients in the cSSSI studies with baseline creatinine clearance ≤50 mL/min had lower clinical cure rates. Consider these data when selecting antibacterial therapy in patients with baseline moderate/severe renal impairment (CrCl ≤50 mL/min).
Dosage adjustment is required in patients with ≤50 mL/min renal impairment. There is insufficient information to make specific dosage adjustment recommendations for patients with end-stage renal disease (CrCl <10 mL/min), including patients receiving hemodialysis.
Hydroxypropyl-beta-cyclodextrin is excreted in urine and may accumulate in patients with renal impairment. Serum creatinine should be closely monitored and, if renal toxicity is suspected, an alternative agent should be considered.
Hepatic Impairment
The HABP/VABP and cSSSI trials included patients with normal hepatic function and with hepatic impairment. No dosage adjustment is recommended in patients with mild or moderate hepatic impairment.
Females of Reproductive Potential and Males
Telavancin did not affect the fertility or reproductive performance of adult male rats (exposed to telavancin for at least 4 weeks prior to mating) or female rats (exposed to telavancin for at least 2 weeks prior to mating).
Male rats given telavancin for 6 weeks, at exposures similar to those measured in clinical studies, displayed altered sperm parameters that were reversible following an 8-week recovery period.
Immunocompromised Patients
There is no FDA guidance one the use of Telavancin hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Telavancin hydrochloride Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Telavancin hydrochloride and IV administrations.
Overdosage
In the event of overdosage, VIBATIV should be discontinued and supportive care is advised with maintenance of glomerular filtration and careful monitoring of renal function. Following administration of a single dose of VIBATIV 7.5 mg/kg to subjects with end-stage renal disease, approximately 5.9% of the administered dose of telavancin was recovered in the dialysate following 4 hours of hemodialysis. However, no information is available on the use of hemodialysis to treat an overdosage.
The clearance of telavancin by continuous venovenous hemofiltration (CVVH) was evaluated in an in vitro study. Telavancin was cleared by CVVH and the clearance of telavancin increased with increasing ultrafiltration rate. However, the clearance of telavancin by CVVH has not been evaluated in a clinical study; thus, the clinical significance of this finding and use of CVVH to treat an overdosage is unknown.
Pharmacology
There is limited information regarding Telavancin hydrochloride Pharmacology in the drug label.
Mechanism of Action
Telavancin inhibits cell wall biosynthesis by binding to late-stage peptidoglycan precursors, including lipid II. Telavancin also binds to the bacterial membrane and disrupts membrane barrier function.
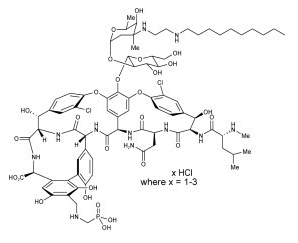
Structure
Telavancin hydrochloride has the following chemical structure:
Pharmacodynamics
There is limited information regarding Telavancin hydrochloride Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Telavancin hydrochloride Pharmacokinetics in the drug label.
Nonclinical Toxicology
Interactions with Other Antibacterial Drugs
In vitro investigations demonstrated no antagonism between telavancin and amikacin, aztreonam, cefepime, ceftriaxone, ciprofloxacin, gentamicin, imipenem, meropenem, oxacillin, piperacillin/tazobactam, rifampin, and trimethoprim/sulfamethoxazole when tested in various combinations against telavancin-susceptible staphylococci, streptococci, and enterococci. This information is not available for other bacteria.
Cross-Resistance
Some vancomycin-resistant enterococci have a reduced susceptibility to telavancin. There is no known cross-resistance between telavancin and other classes of antibacterial drugs.
Antibacterial Activity
Telavancin has been shown to be active against most isolates of the following microorganisms both in vitro and in clinical infections as described in the Indications and Usage section:
- Facultative Gram-Positive Microorganisms
- Staphylococcus aureus (including methicillin-resistant isolates)
- Enteroc occus faecalis (vancomycin-susceptible isolates only)
- Streptococcus agalactiae
- Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus)
- Streptococcus pyogenes
Greater than 90% of the following microorganisms exhibit an in vitro MIC less than or equal to the telavancin-susceptible breakpoint for organisms of similar genus shown in TABLE 9. The safety and effectiveness of telavancin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
- Facultative Gram-Positive Microorganisms
- Enterococcus faecium (vancomycin-susceptible isolates only)
- Staphylococcus haemolyticus
- Streptococcus dysgalactiae subsp. equisimilis
- Staphylococcus epidermidis
Carcinogenesis and Mutagenesis
Long-term studies in animals to determine the carcinogenic potential of telavancin have not been performed.
Neither mutagenic nor clastogenic potential of telavancin was found in a battery of tests including: assays for mutagenicity (Ames bacterial reversion), an in vitro chromosome aberration assay in human lymphocytes, and an in vivo mouse micronucleus assay.
Animal Toxicology and/or Pharmacology
Two-week administration of telavancin in rats produced minimal renal tubular vacuolization with no changes in BUN or creatinine. These effects were not seen in studies conducted in dogs for similar duration. Four weeks of treatment resulted in reversible elevations in BUN and/or creatinine in association with renal tubular degeneration that further progressed following 13 weeks of treatment.
These effects occurred at exposures (based on AUCs) that were similar to those measured in clinical trials.
The potential effects of continuous venovenous hemofiltration (CVVH) on the clearance of telavancin were examined in an in vitro model using bovine blood. Telavancin was cleared by CVVH and the clearance of telavancin increased with increasing ultrafiltration rate.
Clinical Studies
There is limited information regarding Telavancin hydrochloride Clinical Studies in the drug label.
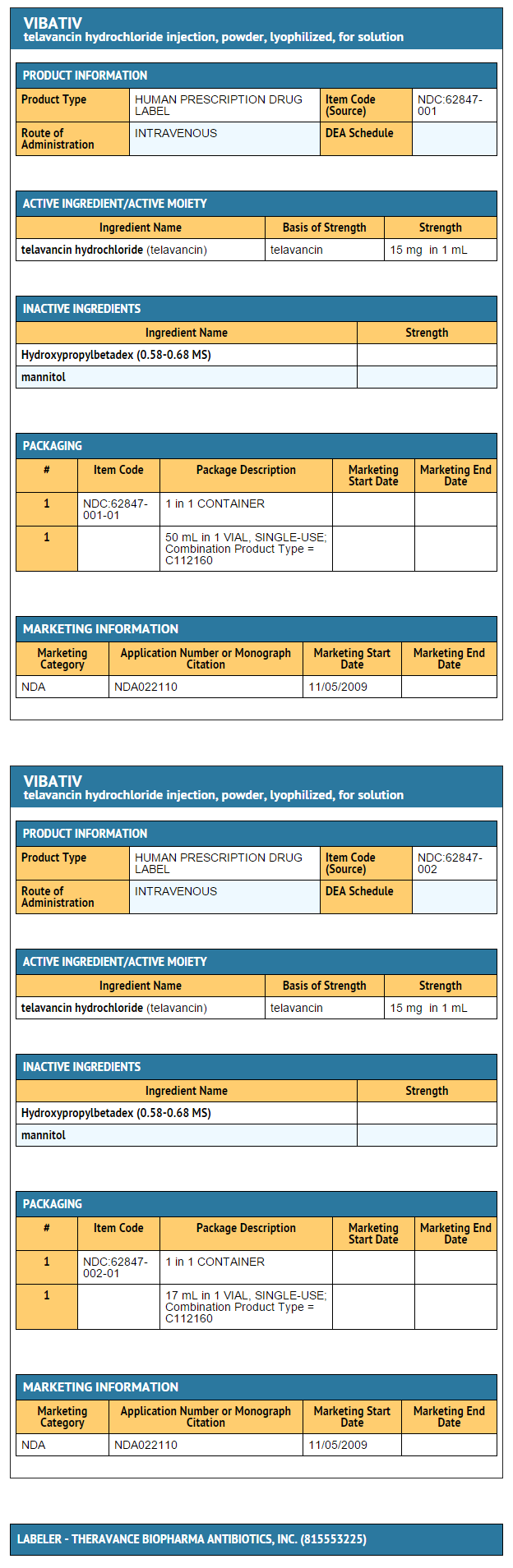
How Supplied
- Cartons of 10 individually packaged 250 mg single-dose vials (NDC 62847-002-01)
- Cartons of 10 individually packaged 750 mg single-dose vials (NDC 62847-001-01)
Storage
Store at refrigerated temperatures of 2 to 8°C (35 to 46 °F).
Images
Drug Images
{{#ask: Page Name::Telavancin hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Telavancin hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Use During Pregnancy and By Women of Childbearing Potential
Women of childbearing potential (those who have not had: complete absence of menses for at least 24 months or medically confirmed menopause, medically confirmed primary ovarian failure, a history of hysterectomy, bilateral oophorectomy, or tubal ligation) should:
- Be informed about the potential risk of fetal harm if VIBATIV is used during pregnancy
- Have a pregnancy test prior to administration of VIBATIV
- If not pregnant, use effective contraceptive methods to prevent pregnancy during VIBATIV treatment
- Notify their prescribing physician/ healthcare provider if they become pregnant during VIBATIV treatment
Pregnancy Registry
There is a pregnancy registry that monitors pregnancy outcomes in women exposed to VIBATIV during pregnancy. Physicians are encouraged to register pregnant patients, or pregnant women may enroll themselves in the pregnancy registry by calling 1-855-633-8479 FREE.
Diarrhea
Diarrhea is a common problem caused by antibiotics that usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as 2 or more months after having received the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Correct Use of Antibacterial Drugs
Patients should be counseled that antibacterial drugs including VIBATIV should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When VIBATIV is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of immediate treatment, and (2) increase the likelihood that the bacteria will develop resistance and will not be treatable by VIBATIV or other antibacterial drugs in the future.
Common Adverse Effects
Patients should be informed about the common adverse effects of VIBATIV including diarrhea, taste disturbance, nausea, vomiting, headache, and foamy urine. Patients should be instructed to inform their healthcare provider if they develop any unusual symptom, or if any known symptom persists or worsens. Patients should be instructed to inform their healthcare provider of any other medications they are currently taking with VIBATIV, including over-the-counter medications.
Precautions with Alcohol
Alcohol-Telavancin hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Vibativ
Look-Alike Drug Names
There is limited information regarding Telavancin hydrochloride Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.