Taliglucerase alfa
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Taliglucerase alfa is a {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- ELELYSO is indicated for long-term enzyme replacement therapy (ERT) for adult and pediatric patients with a confirmed diagnosis of Type 1 Gaucher disease.
Dosage
Recommended Dosage
- Treatment-naïve patients: The recommended dosage for treatment-naïve adult and pediatric patients 4 years of age and older is 60 units per kg of body weight administered every other week as a 60 to 120 minute intravenous infusion.
- Patients switching from imiglucerase: Patients currently being treated with imiglucerase for Type 1 Gaucher disease can be switched to ELELYSO. Patients previously treated on a stable dosage of imiglucerase are recommended to begin treatment with ELELYSO at that same dosage when they switch from imiglucerase to ELELYSO Dosage adjustments can be made based on achievement and maintenance of each patient's therapeutic goals.
- ELELYSO should be reconstituted, diluted, and administered under the supervision of a healthcare professional.
DOSAGE FORMS AND STRENGTHS
- For injection: lyophilized powder for reconstitution; 200 units/vial.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Taliglucerase alfa in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Taliglucerase alfa in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Taliglucerase alfa in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Taliglucerase alfa in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Taliglucerase alfa in pediatric patients.
Contraindications
- None
Warnings
Hypersensitivity Reactions Including Anaphylaxis
- Serious hypersensitivity reactions, including anaphylaxis, have occurred in some patients treated with ELELYSO. In clinical trials, 2 of 72 (2.8%) patients treated with ELELYSO experienced signs and symptoms consistent with anaphylaxis. Signs and symptoms of these patients included urticaria, hypotension, flushing, wheezing, chest tightness, nausea, vomiting, and dizziness. These reactions occurred during ELELYSO infusion.
- In clinical trials with ELELYSO, 21 of 72 (29%) patients experienced hypersensitivity reactions, including anaphylaxis. Signs and symptoms of hypersensitivity reactions included pruritus, angioedema, flushing, erythema, rash, nausea, vomiting, cough, chest tightness, and throat irritation. These reactions have occurred up to 3 hours after the start of infusion.
- Due to the potential for anaphylaxis, appropriate medical support should be readily available when ELELYSO is administered. Observe patients closely for an appropriate period of time after administration of ELELYSO, taking into account the time to onset of anaphylaxis seen in clinical trials. Inform patients of the signs and symptoms of anaphylaxis, and instruct them to seek immediate medical care should signs and symptoms occur. If anaphylaxis occurs, ELELYSO should be immediately discontinued, and appropriate medical treatment should be initiated.
- Management of hypersensitivity reactions should be based on the severity of the reaction and include slowing or temporary interruption of the infusion and/or administration of antihistamines, antipyretics, and/or corticosteroids for mild reactions. Pretreatment with antihistamines and/or corticosteroids may prevent subsequent hypersensitivity reactions. Patients were not routinely premedicated prior to infusion of ELELYSO during clinical studies. If severe hypersensitivity reactions occur, immediately stop the infusion of ELELYSO and initiate appropriate treatment.
- Consider the risks and benefits of re-administering ELELYSO in patients who have experienced a severe reaction associated with ELELYSO. Caution should be exercised upon rechallenge, and appropriate medical support should be readily available.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In the clinical trials with ELELYSO, either as initial therapy or as therapy following a switch from imiglucerase (N=72), the most common (≥ 5%) adverse reactions included pruritus, flushing, headache, arthralgia, pain in extremity, abdominal pain, vomiting, fatigue, back pain, dizziness, nausea, and rash.
Clinical Trials of ELELYSO as Initial Therapy
- Clinical Trial in Patients 19 Years and Older
- The safety of ELELYSO at dosages of either 30 units/kg (n=16) or 60 units/kg (n=16) every other week was assessed in 32 adult treatment-naïve patients (aged 19 to 74 years) with Type 1 Gaucher disease in a 9-month randomized clinical trial.

- Similar adverse reactions were observed in patients who continued ELELYSO treatment during the extension trial for up to 24 months. One patient experienced a mild and intermittent Type III immune-mediated fixed drug eruption and continued in the study.
- Clinical Trial in Patients 16 Years and Younger
- The safety of ELELYSO at dosages of either 30 units/kg (n=4) or 60 units/kg (n=5) every other week was assessed in 9 pediatric treatment-naïve patients (aged 2 to 13 years) with Type 1 Gaucher disease in a 12-month randomized clinical trial.
- The most common adverse reaction (≥10%) was vomiting, which occurred in 4 of 9 patients. Two patients developed hypersensitivity reactions; one patient experienced severe vomiting and gastrointestinal inflammation, and 1 experienced mild throat irritation and chest discomfort. Both patients responded to treatment with antihistamines and continued ELELYSO treatment.
Clinical Trial in Patients Switching from Imiglucerase Treatment to ELELYSO
- The safety of ELELYSO was assessed in 31 patients (26 adult and 5 pediatric patients), ages 6 to 66 years old, with Type 1 Gaucher disease who had previously been receiving treatment with imiglucerase for a minimum of 2 years. ELELYSO was administered for 9 months at the same number of units as each patient's previous imiglucerase dose.
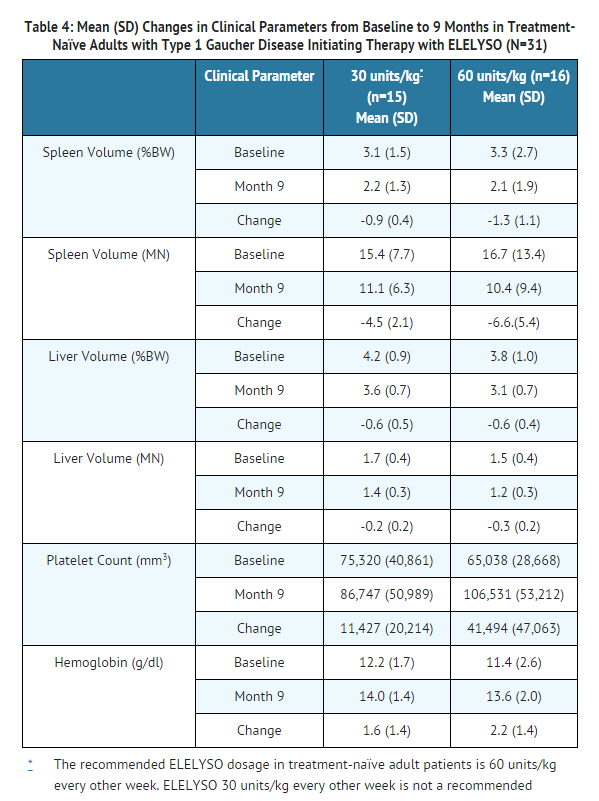
Immunogenicity
- As with all therapeutic proteins, patients may develop anti-drug antibodies (ADA) to ELELYSO.
- In clinical trials of treatment-naïve adults, 17 (53%) of 32 patients developed ADA during treatment with ELELYSO, and 2 (6%) of 32 patients tested positive for ADA at baseline prior to ELELYSO treatment. Of the 17 patients who developed ADA during ELELYSO treatment, 6 patients (35%) developed hypersensitivity reactions, 2 of whom met criteria for anaphylaxis. Two of the 17 patients who developed ADA during ELELYSO treatment discontinued treatment due to hypersensitivity reactions, one of whom had met criteria for anaphylaxis. Of the 2 patients who tested positive for ADA prior to initiation of ELELYSO treatment, one patient developed a hypersensitivity reaction during the first dose of ELELYSO and withdrew from the study. The second patient did not experience an adverse reaction.
- In a clinical trial of treatment-naïve pediatric patients, 2 (22%) of 9 patients developed ADA during treatment with ELELYSO, and one of 9 patients was ADA-positive prior to initiation of ELELYSO. Two patients (1 who developed ADA during treatment and 1 who was ADA-positive at baseline) experienced hypersensitivity reactions. Both patients continued treatment with ELELYSO.
- In a clinical trial of 31 patients (26 adult and 5 pediatric patients) who switched from imiglucerase to ELELYSO treatment, 4 adults (13% of patients) developed ADA during treatment with ELELYSO. Four additional patients (13%, 2 adults and 2 children) tested positive for ADA at baseline but became ADA-negative after the switch to ELELYSO. Two adult patients (1 patient who developed ADA after the switch and 1 who was ADA positive at baseline) experienced hypersensitivity reactions. Both patients continued treatment with ELELYSO.
- The relationship between ADA and hypersensitivity reactions is not fully understood. Monitoring for ADA to ELELYSO may be useful in ADA positive patients or in patients who have experienced hypersensitivity reactions to ELELYSO or other enzyme replacement therapies.
- Twenty-nine of the 30 adult and pediatric patients who tested positive for ADA were tested for neutralizing antibodies capable of inhibiting the enzymatic activity of ELELYSO. Neutralizing antibodies were detected in 3 (10.3%) of 29 patients, 2 treatment-naïve adult patients and 1 adult patient who switched from imiglucerase. Due to limited available data, it is not possible to determine a relationship between the presence of neutralizing antibodies and therapeutic response with ELELYSO.
- Immunogenicity assay results are highly dependent on the sensitivity and specificity of the assay and may be influenced by several factors such as: assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to ELELYSO with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of ELELYSO in countries where it is marketed. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: Anaphylaxis
Drug Interactions
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Taliglucerase alfa in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Taliglucerase alfa during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Taliglucerase alfa with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Taliglucerase alfa with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Taliglucerase alfa with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Taliglucerase alfa with respect to specific gender populations.
Race
There is no FDA guidance on the use of Taliglucerase alfa with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Taliglucerase alfa in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Taliglucerase alfa in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Taliglucerase alfa in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Taliglucerase alfa in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Taliglucerase alfa in the drug label.
- Description
IV Compatibility
Preparation Instructions
- Each vial of ELELYSO provides 200 units of taliglucerase alfa and is intended for single use only. Do not use the vial more than one time. The reconstitution and dilution steps must be completed using aseptic technique.
- ELELYSO should be reconstituted with Sterile Water for Injection and diluted with 0.9% Sodium Chloride Injection, USP, to a final volume of 100 mL to 200 mL, and delivered by intravenous infusion.
- Prepare ELELYSO according to the following steps. Use aseptic technique.
- Determine the number of vials to be reconstituted based on the patient's weight and the recommended dose of 60 units/kg, using the following calculations (1–3):
- Total dose in units = Patient's weight (kg) × dose (units/kg)
- Total number of vials = Total dose in units divided by 200 units/vial
- Round up to the next whole vial.
- Remove the required number of vials from the refrigerator. Do not leave these vials at room temperature longer than 24 hours prior to reconstitution. Do not heat or microwave these vials.
- Reconstitute each vial of ELELYSO with 5.1 mL of Sterile Water for Injection to yield a reconstituted product volume of 5.3 mL and a withdrawal volume of 5 mL. Upon reconstitution, mix vials gently. DO NOT SHAKE. Prior to further dilution, visually inspect the solution in the vials; the solution should be clear and colorless. Do not use if the solution is discolored or if foreign particulate matter is present.
Withdraw the calculated dose of drug from the appropriate number of vials and dilute with 0.9% Sodium Chloride Injection, USP, to a final volume of 100 to 200 mL.
- For pediatric patients, a final volume of 100 to 120 mL should be used.
- For adult patients, a final volume of 130 to 150 mL may be used. However, if the volume of reconstituted product alone is equal to or greater than 130 to 150 mL, then the final volume should not exceed 200 mL.
- Mix gently. DO NOT SHAKE. Since this is a protein solution, slight flocculation (described as translucent fibers) occurs occasionally after dilution.
Overdosage
There is limited information regarding Chronic Overdose of Taliglucerase alfa in the drug label.
Pharmacology
There is limited information regarding Taliglucerase alfa Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Taliglucerase alfa Mechanism of Action in the drug label.
Structure
There is limited information regarding Taliglucerase alfa Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Taliglucerase alfa in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Taliglucerase alfa in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Taliglucerase alfa in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Taliglucerase alfa in the drug label.
How Supplied
Storage
There is limited information regarding Taliglucerase alfa Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Taliglucerase alfa |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Taliglucerase alfa |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Taliglucerase alfa in the drug label.
Precautions with Alcohol
- Alcohol-Taliglucerase alfa interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Taliglucerase alfa
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Taliglucerase alfa |Label Name=Taliglucerase alfa11.png
}}
{{#subobject:
|Label Page=Taliglucerase alfa |Label Name=Taliglucerase alfa11.png
}}