Takayasu's arteritis: Difference between revisions
Rim Halaby (talk | contribs) |
Rim Halaby (talk | contribs) No edit summary |
||
| Line 28: | Line 28: | ||
==Historical Perspective== | ==Historical Perspective== | ||
The first case of Takayasu’s arteritis was described in 1908 by Dr. [[Mikito Takayasu]] at the Annual Meeting of the Japan Ophthalmology Society.<ref>{{WhoNamedIt|synd|2722}}</ref><ref>M. Takayasu. A case with peculiar changes of the central retinal vessels. Acta Societatis ophthalmologicae Japonicae, Tokyo 1908, 12: 554.</ref> Dr. Takayasu described a peculiar "wreathlike" appearance of [[blood vessel]]s in the back of the eye ([[retina]]). Two Japanese colleagues at the same meeting (Dr. Onishi and Dr. Kagoshima) reported similar eye findings in patients whose [[Pulse|wrist pulse]]s were absent. It is now known that the blood vessel malformations that occur in the retina are a response ([[angiogenesis|new blood vessel growth]]) to arterial narrowings in the neck, and that the absence of pulses noted in some patients occur because of narrowings of blood vessels to the arms. The eye findings described by Takayasu are rarely seen in patients from North America. | The first case of Takayasu’s arteritis was described in 1908 by Dr. [[Mikito Takayasu]] at the Annual Meeting of the Japan Ophthalmology Society.<ref>{{WhoNamedIt|synd|2722}}</ref><ref>M. Takayasu. A case with peculiar changes of the central retinal vessels. Acta Societatis ophthalmologicae Japonicae, Tokyo 1908, 12: 554.</ref> Dr. Takayasu described a peculiar "wreathlike" appearance of [[blood vessel]]s in the back of the eye ([[retina]]). Two Japanese colleagues at the same meeting (Dr. Onishi and Dr. Kagoshima) reported similar eye findings in patients whose [[Pulse|wrist pulse]]s were absent. It is now known that the blood vessel malformations that occur in the retina are a response ([[angiogenesis|new blood vessel growth]]) to arterial narrowings in the neck, and that the absence of pulses noted in some patients occur because of narrowings of blood vessels to the arms. The eye findings described by Takayasu are rarely seen in patients from North America. | ||
==Classification== | |||
Four types of late-phase Takayasu arteritis are described on the basis of the sites of involvement as follows:<ref>{{cite web |url=http://www.emedicine.com/radio/topic51.htm |title=eMedicine - Arteritis, Takayasu : Article by Robert L Cirillo, Jr, MD, MBA |accessdate=2007-07-19 |format= |work=}}</ref> | |||
* Type I - Classic pulseless type that involves the brachiocephalic trunk, carotid arteries, and subclavian arteries | |||
* Type II - Combination of type I and III | |||
* Type III - Atypical coarctation type that involves the thoracic and abdominal aortas distal to the arch and its major branches | |||
* Type IV - Dilated type that involves extensive dilatation of the length of the aorta and its major branches | |||
==Pathophysiology== | |||
Although its [[etiology|cause]] is unknown, the condition is characterized by segmental and patchy [[granuloma]]tous [[inflammation]] of the aorta and its major derivative branches. This inflammation leads to arterial [[stenosis]], [[thrombosis]], and [[aneurysm]]s. There is also irregular fibrosis of the blood vessels due to chronic vasculitis, leading to sometimes massive intimal fibrosis (fibrosis of the inner section of the blood vessels). Prominent narrowing due to inflammation, granuloma, and fibrosis is often seen in arterial studies such as [[magnetic resonance angiography]] (MRA), [[computed tomography angiography]] (CTA), or arterial [[angiography]] (DSA). | |||
The genetic contribution to the pathogenesis of Takayasu's arteritis is supported by the genetic association with HLA-B∗52. A recent large collaborative study uncovered multiple additional susceptibility loci for this disease, increasing the number of genetic loci for this disease to five risk loci across the genome.<ref name=SARUHAN2013>{{cite journal|last=Saruhan-Direskeneli|first=G|coauthors=Hughes, T; Aksu, K; Keser, G; Coit, P; Aydin, SZ; Alibaz-Oner, F; Kamalı, S; Inanc, M; Carette, S; Hoffman, GS; Akar, S; Onen, F; Akkoc, N; Khalidi, NA; Koening, C; Karadag, O; Kiraz, S; Langford, CA; McAlear, CA; Ozbalkan, Z; Ates, A; Karaaslan, Y; Maksimowicz-McKinnon, K; Monach, PA; Ozer, HT; Seyahi, E; Fresko, I; Cefle, A; Seo, P; Warrington, KJ; Ozturk, MA; Ytterberg, SR; Cobankara, V; Onat, AM; Guthridge, JM; James, JA; Tunc, E; Duzgun, N; Bıcakcıgil, M; Yentür, SP; Merkel, PA; Direskeneli, H; Sawalha, AH|title=Identification of Multiple Genetic Susceptibility Loci in Takayasu Arteritis|journal=American journal of human genetics|date=Jul 2, 2013|pmid=23830517|volume=93|issue=2|pages=298–305|doi=10.1016/j.ajhg.2013.05.026|pmc=3738826}}</ref> About 200,000 genetic variants were genotyped in two ethnically divergent Takayasu's arteritis cohorts from Turkey and North America by using a custom-designed genotyping platform (Immunochip). Additional genetic variants and the classical HLA alleles were imputed and analyzed. The study identified and confirmed two independent susceptibility loci within the HLA region (r2 < 0.2): HLA-B/MICA (rs12524487, OR = 3.29, p = 5.57 × 10-16) and HLA-DQB1/HLA-DRB1 (rs113452171, OR = 2.34, p = 3.74 × 10-9; and rs189754752, OR = 2.47, p = 4.22 × 10-9). In addition, a genetic association was identified and confirmed between Takayasu's arteritis and the FCGR2A/FCGR3A locus on chromosome 1 (rs10919543, OR = 1.81, p = 5.89 × 10-12). The risk allele in this locus results in increased mRNA expression of FCGR2A. In addition, a genetic association between IL12B and Takayasu arteritis was established (rs56167332, OR = 1.54, p = 2.18 × 10-8). A fifth genetic locus for the disease on chromosome 21q22 downstream of PSMG1 was also revealed (P=4.39X10-7).<ref name="SARUHAN2013"/> | |||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
| Line 48: | Line 62: | ||
Five year survival in TA is over 90%, but TA is associated with significant morbidity. | Five year survival in TA is over 90%, but TA is associated with significant morbidity. | ||
== | ==Symptoms== | ||
The disease can be divided into two phases; the Initial phase is pre-pulseless phase, in which patients presents which non-specific constitutional symptoms of [[vasculitis]], which may include any of the following: | |||
* [[Fatigue]] | |||
* [[Fever of unknown origin]] | |||
* [[Weight loss]] | |||
* [[Myalgia]] | |||
* [[Arthralgia]] | |||
With progression of the disease and involvement of the branches of aorta,<ref name="pmid7909656">{{cite journal| author=Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M et al.| title=Takayasu arteritis. | journal=Ann Intern Med | year= 1994 | volume= 120 | issue= 11 | pages= 919-29 | pmid=7909656 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7909656 }} </ref> the specific signs appear secondary to narrowing/occlusion of the branches of aorta. | |||
* Involvement of [[subclavian artery]] is common and leads to claudication of upper extremities (pain with activity). The stenosis of subclavian artery sometimes leads to [[subclavian steel syndrome]]<ref name="pmid15335">{{cite journal| author=Yoneda S, Nukada T, Tada K, Imaizumi M, Takano T| title=Subclavian steal in Takayasu's arteritis. A hemodynamic study by means of ultrasonic Doppler flowmetry. | journal=Stroke | year= 1977 | volume= 8 | issue= 2 | pages= 264-8 | pmid=15335 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15335 }} </ref>. In this phenomenon, the stenosis of subclavian artery, proximal to the origin of Vertebral Artery leads to retrograde flow of blood from the vertebral artery back to subclavian artery during exercise, secondary to vasodilation of blood vessels. The retrograde flow of blood from the vertebral artery back towards subclavian compromises blood flow in [[posterior cerebral bed]], leading to various neurological symptoms including presyncope/syncope. | |||
* | * Involvement of carotid and vertebral arteries: headache, vertigo, syncope, convulsions and dementia | ||
* Involvement of coronary arteries: chest pain, angina which may progress to myocardial infarction | |||
* Involvement of ascending aorta may: aortic regurgiatation | |||
* Skin lesions resemble erythema nodosum, erythema multiforme, pyoderma gangrenosum | |||
In rare instances, the disease may involve abdominal, pulmonary vessels. In advance stages of the disease, the occlusion of the vessels to the extremities may can ischemic ulcerations. Due to chronic nature of the disease, collateral circulation develops in the affected area. | |||
==Diagnosis== | ==Diagnosis== | ||
| Line 110: | Line 132: | ||
[[File:Takayasu arteritis.JPG|300px]] | [[File:Takayasu arteritis.JPG|300px]] | ||
===Angiography== | ====Angiography==== | ||
==Treatment== | ==Treatment== | ||
Revision as of 17:08, 2 February 2015
Template:DiseaseDisorder infobox Template:Search infobox
For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Synonyms and keywords: Aortic arch syndrome, nonspecific aortoarteritis, pulseless disease
Overview
Takayasu's arteritis is an inflammatory disease of unknown etiology that affects mainly the aorta and its branches. Takayasu's arteritis is a form of large vessel granulomatous vasculitis characterized by massive intimal fibrosis and vascular narrowing. Due to obstruction of the main branches of the aorta, including the left common carotid artery, the brachiocephalic artery, and the left subclavian artery, Takayasu's arteritis can present as pulseless upper extremities (arms, hands, and wrists with weak or absent pulses on the physical examination) which may be why it is also commonly referred to as the "pulseless disease".
Historical Perspective
The first case of Takayasu’s arteritis was described in 1908 by Dr. Mikito Takayasu at the Annual Meeting of the Japan Ophthalmology Society.[1][2] Dr. Takayasu described a peculiar "wreathlike" appearance of blood vessels in the back of the eye (retina). Two Japanese colleagues at the same meeting (Dr. Onishi and Dr. Kagoshima) reported similar eye findings in patients whose wrist pulses were absent. It is now known that the blood vessel malformations that occur in the retina are a response (new blood vessel growth) to arterial narrowings in the neck, and that the absence of pulses noted in some patients occur because of narrowings of blood vessels to the arms. The eye findings described by Takayasu are rarely seen in patients from North America.
Classification
Four types of late-phase Takayasu arteritis are described on the basis of the sites of involvement as follows:[3]
- Type I - Classic pulseless type that involves the brachiocephalic trunk, carotid arteries, and subclavian arteries
- Type II - Combination of type I and III
- Type III - Atypical coarctation type that involves the thoracic and abdominal aortas distal to the arch and its major branches
- Type IV - Dilated type that involves extensive dilatation of the length of the aorta and its major branches
Pathophysiology
Although its cause is unknown, the condition is characterized by segmental and patchy granulomatous inflammation of the aorta and its major derivative branches. This inflammation leads to arterial stenosis, thrombosis, and aneurysms. There is also irregular fibrosis of the blood vessels due to chronic vasculitis, leading to sometimes massive intimal fibrosis (fibrosis of the inner section of the blood vessels). Prominent narrowing due to inflammation, granuloma, and fibrosis is often seen in arterial studies such as magnetic resonance angiography (MRA), computed tomography angiography (CTA), or arterial angiography (DSA).
The genetic contribution to the pathogenesis of Takayasu's arteritis is supported by the genetic association with HLA-B∗52. A recent large collaborative study uncovered multiple additional susceptibility loci for this disease, increasing the number of genetic loci for this disease to five risk loci across the genome.[4] About 200,000 genetic variants were genotyped in two ethnically divergent Takayasu's arteritis cohorts from Turkey and North America by using a custom-designed genotyping platform (Immunochip). Additional genetic variants and the classical HLA alleles were imputed and analyzed. The study identified and confirmed two independent susceptibility loci within the HLA region (r2 < 0.2): HLA-B/MICA (rs12524487, OR = 3.29, p = 5.57 × 10-16) and HLA-DQB1/HLA-DRB1 (rs113452171, OR = 2.34, p = 3.74 × 10-9; and rs189754752, OR = 2.47, p = 4.22 × 10-9). In addition, a genetic association was identified and confirmed between Takayasu's arteritis and the FCGR2A/FCGR3A locus on chromosome 1 (rs10919543, OR = 1.81, p = 5.89 × 10-12). The risk allele in this locus results in increased mRNA expression of FCGR2A. In addition, a genetic association between IL12B and Takayasu arteritis was established (rs56167332, OR = 1.54, p = 2.18 × 10-8). A fifth genetic locus for the disease on chromosome 21q22 downstream of PSMG1 was also revealed (P=4.39X10-7).[4]
Epidemiology and Demographics
Race
Although Takayasu's arteritis has been reported worldwide, there is a predilection for young Asian women. In the Western world, atherosclerosis is a more frequent cause of obstruction of the aortic arch vessels than is Takayasu's arteritis.
Age
The age of onset is typically between 15 and 30 years.
Gender
Females are about 8–9 times more likely to be affected by Takayasu's arteritis than males.
Natural History, Complications and Prognosis
Natural History
Those with the disease often notice symptoms between 15 and 30 years of age. Some people develop an initial "inflammatory phase" characterized by systemic illness with signs and symptoms of malaise, fever, night sweats, weight loss, joint pain, fatigue, and fainting. Fainting may result from subclavian steal syndrome or carotid sinus hypersensitivity.[5] There is also often anemia and marked elevation of the ESR or C-reactive protein (nonspecific markers of inflammation). The initial "inflammatory phase" is often followed by a secondary "pulseless phase". The "pulseless phase" is characterized by vascular insufficiency from intimal narrowing of the vessels manifesting as arm or leg claudication, renal artery stenosis causing hypertension, and neurological manifestations due to decreased blood flow to the brain. Of note is the function of renal artery stenosis in causation of high blood pressure: Normally perfused kidneys produce proportionate amount of a substance called renin. Stenosis of the renal arteries causes hypo-perfusion (decreased blood flow) of the juxtaglomerular apparatus, resulting in exaggerated secretion of renin, and high blood levels of aldosterone, eventually leading to water and salt retention and high blood pressure. The neurological symptoms of the disease vary depending on the degree, and the nature of the blood vessel obstruction and can range from lightheadedness, to seizures in severe cases. One rare but important feature of the Takayasu's arteritis is ocular involvement in form of visual field defects, vision loss, or retinal hemorrhage. Some individuals with Takayasu's arteritis may present with only late vascular changes, without a preceding systemic illness. In the late stage, weakness of the arterial walls may give rise to localized aneurysms. As with all aneurysms, possibility of rupture and vascular bleeding is existent and requires monitoring. Raynaud's phenomenon is commonly found in this disease, mainly due to decreased circulation of the blood to the arms.
Complications
Cardiac complications of takayasu arteritis may include aortic regurgitation, myocarditis and congestive heart failure.
Prognosis
Five year survival in TA is over 90%, but TA is associated with significant morbidity.
Symptoms
The disease can be divided into two phases; the Initial phase is pre-pulseless phase, in which patients presents which non-specific constitutional symptoms of vasculitis, which may include any of the following:
With progression of the disease and involvement of the branches of aorta,[6] the specific signs appear secondary to narrowing/occlusion of the branches of aorta.
- Involvement of subclavian artery is common and leads to claudication of upper extremities (pain with activity). The stenosis of subclavian artery sometimes leads to subclavian steel syndrome[7]. In this phenomenon, the stenosis of subclavian artery, proximal to the origin of Vertebral Artery leads to retrograde flow of blood from the vertebral artery back to subclavian artery during exercise, secondary to vasodilation of blood vessels. The retrograde flow of blood from the vertebral artery back towards subclavian compromises blood flow in posterior cerebral bed, leading to various neurological symptoms including presyncope/syncope.
- Involvement of carotid and vertebral arteries: headache, vertigo, syncope, convulsions and dementia
- Involvement of coronary arteries: chest pain, angina which may progress to myocardial infarction
- Involvement of ascending aorta may: aortic regurgiatation
- Skin lesions resemble erythema nodosum, erythema multiforme, pyoderma gangrenosum
In rare instances, the disease may involve abdominal, pulmonary vessels. In advance stages of the disease, the occlusion of the vessels to the extremities may can ischemic ulcerations. Due to chronic nature of the disease, collateral circulation develops in the affected area.
Diagnosis
Initially, a diagnosis of TA can be missed for months or years because its symptoms can be nonspecific (fever, lethargy, etc.). The American College of Rheumatology (ACR) has established diagnostic criteria for Takayasu arteritis. The patient needs to meet 3 out of 6 criteria for the diagnosis of Takayasu's arteritis:[8]
- Age at the onset of disease <40 years
- Claudication of the extremities
- Decreased or absent brachial artery pulse in one or both arms
- Difference of at least 10 mmHg in systolic blood pressure between the arms
- Bruit over either one or both subclavian arteries or abdominal artery
- Evidence of narrowing of aorta or its primary branches on arteriography, not due to atherosclerosis, fibromuscular dyaplasia, or other causes
Physical Examination
While Takayasu arteritis is sometimes referred to as the "pulseless disease", it is important to note that a missing peripheral pulse usually occurs late in the course of TA. More often individuals with TA present with an asymmetric pulse.[9]
Imaging Studies
The diagnosis of TA is sometimes made when a widened mediastinum is noted on a chest x ray. A follow-up CT scan will often demonstrate a widened aortic arch. [9]
Computed Tomography and Magnetic Resonance Angiography
Computed Tomography (CT) and Magnetic Resonance Angiography (MRA) are the gold standrad imaging modalities for TA and may demonstrate mural thickening of the aorta and luminal narrowing. Use of contrast may reveal inflammatory lesions prior to the development of stenoses; these lesions may be missed by angiography. Aortic lesions including stenosis, dilatation, wall thickening, and mural thrombi are well visualized on MRI, which is less adequate in visualizing distal lesions of the subclavian vessels and common carotids. [10] Non-contrast T2-weighted STIR images may be used to monitor edema in the aortic wall, which may be a surrogate for inflammation; edema may present more than 94% of patients with clinically active disease. One disadvantage of MRA is that pressure differentials cannot be measured across lesions in which imaging findings regarding their hemodynamic significance are inconclusive.
-
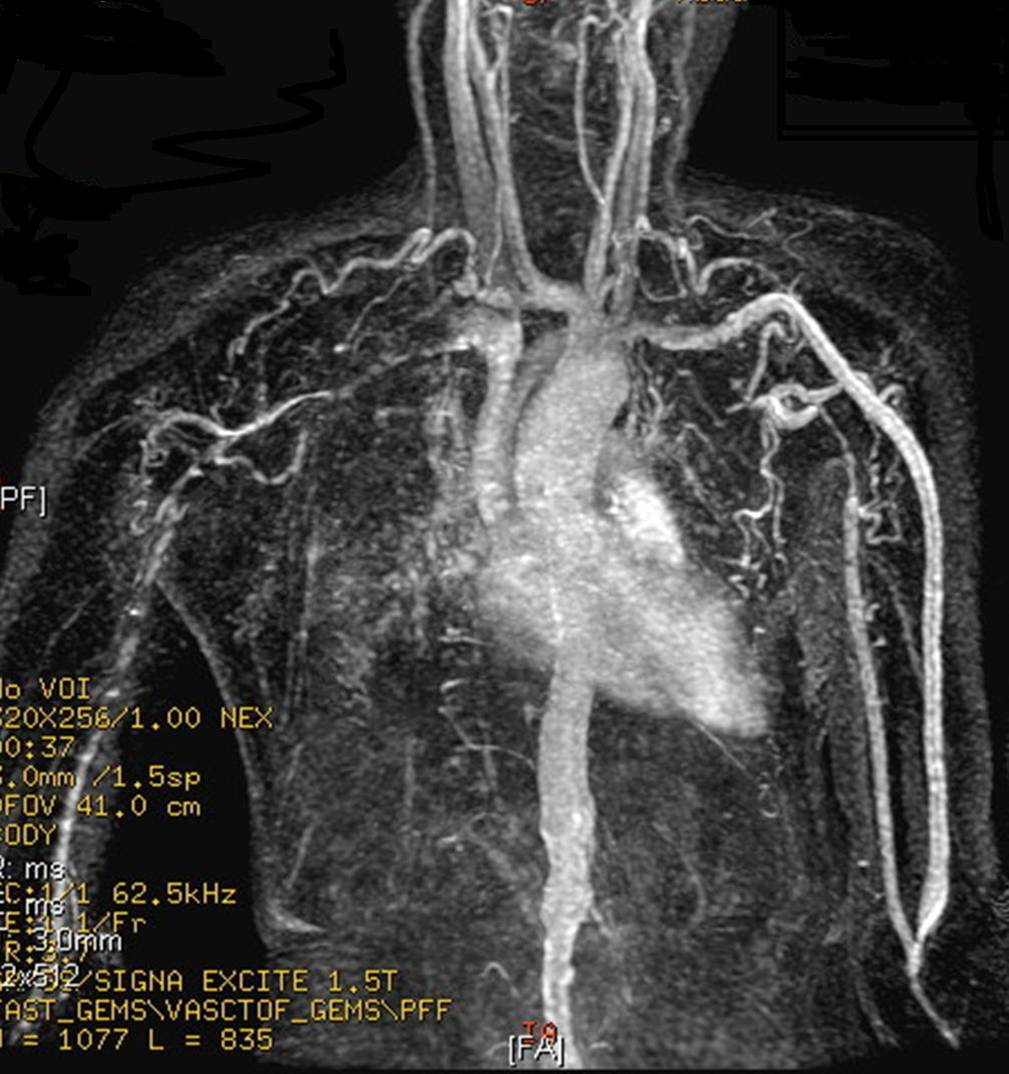
This MR angiogram demonstrates findings typical of Takayasu arteritis. A 32 years old Asian female has CRF and weak functioning iatrogenic fistula in left upper limb. The cause of malfunction can be explained by narrowing and occlusion of upper limb arteries. Notice also narrowed abdominal aorta. (Image courtesy of Dr Ahmed Haroun)
-
MRA: A 15 year-old girl with known Takayasu arteritis presented for MRI to investigate back pain. The axial T1-weighted post-gadolinium MRI above shows thickened, enhancing aortic wall, consistent with large vessel vasculitis. (Image courtesy of Dr Laughlin Dawes)
Warning: Gadolinium-based contrast agents [gadopentetate dimeglumine (Magnevist), gadobenate dimeglumine (MultiHance), gadodiamide (Omniscan), gadoversetamide (OptiMARK), gadoteridol (ProHance)] have recently been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. In December 2006, the FDA had received reports of 90 such cases. Worldwide, over 200 cases have been reported, according to the FDA. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness. [11]
While computed tomography and MRA are the best imaging modalities, findings on other imaging modalities include:
Chest radiography
Chest radiographs may reveal widening of the ascending aorta, irregular descending aorta, aortic calcifications, and rib notching (late findings).
Duplex Doppler
This modality may be used to evaluate and monitor disease in the common carotids and subclavian arteries; however, this imaging study is not useful in evaluating the aorta.
Carotid Ultrasound
Carotid ultrasound demonstrates a homogenous circumferential thickening of the vessel wall that is distinguishable from atherosclerotic thickening.
Gallium-67 radionuclide scan
This scan may demonstrate increased uptake in the aorta and branches.
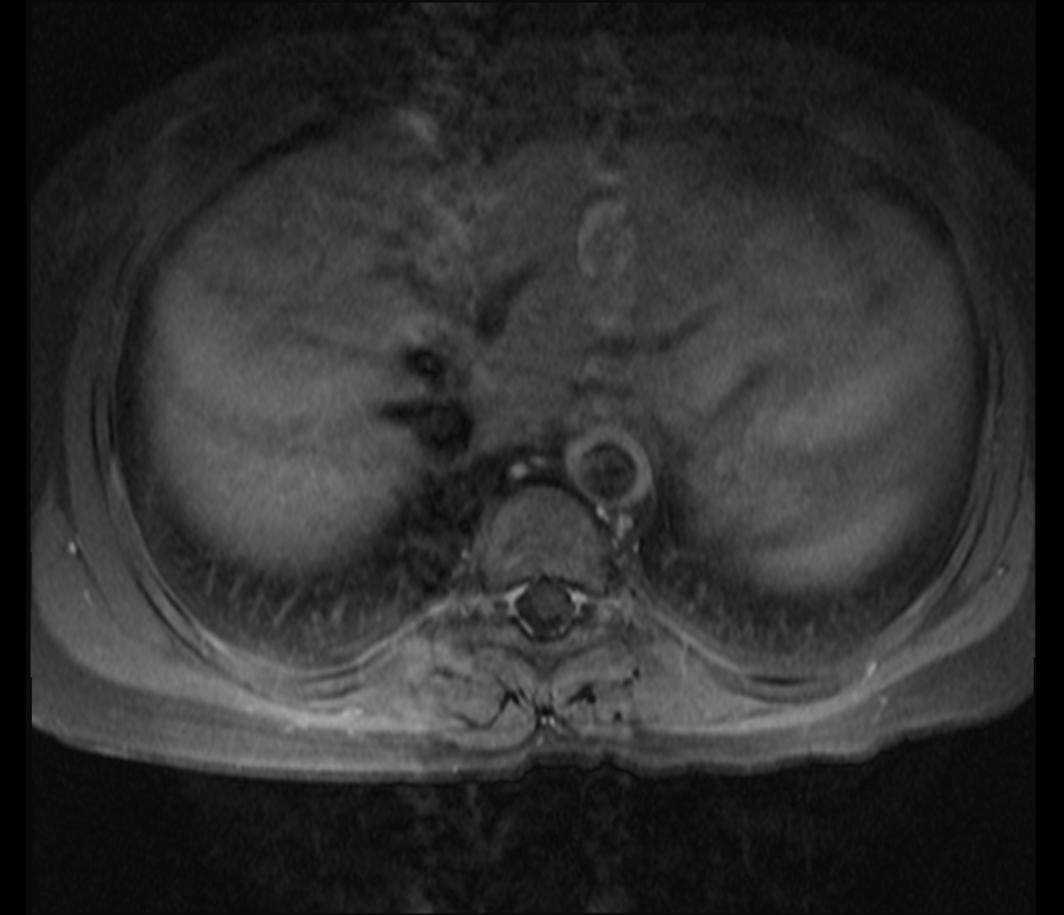
MRI
Shown below is an MRI of a 15 year-old female patient with known Takayasu arteritis, showing thickened, enhancing aortic wall, consistent with large vessel vasculitis.
Angiography
Treatment
Medical Therapy
Most people with Takayasu’s arteritis respond to steroids such as prednisone. The usual starting dose is approximately 1 milligram per kilogram of body weight per day (for most people, this is approximately 60 milligrams a day). Because of the significant side effects of long-term high–dose prednisone use, the starting dose is tapered over several weeks to a dose that the physician feels is tolerable for the patient.
Promising results are achieved with mycophenolate and tocilizumab. If treatment is not kept to a high standard then long term damage or death can occur.
Stress is a major factor that should be avoided at all costs; if this is not taken into account the surge of adrenaline can have a damaging effect on the heart.
Surgery
Surgical options may need to be explored for those who do not respond to steroids. Re-perfusion of tissue can be achieved by large vessel reconstructive surgery such as bypass grafting. Grafting autologous tissue has the highest rates of success. Stenting often obviates the need for surgery.
Percutaneous transluminal coronary angioplasty (PTCA) is not as effective in the long term but has fewer risks.
References
- ↑ Template:WhoNamedIt
- ↑ M. Takayasu. A case with peculiar changes of the central retinal vessels. Acta Societatis ophthalmologicae Japonicae, Tokyo 1908, 12: 554.
- ↑ "eMedicine - Arteritis, Takayasu : Article by Robert L Cirillo, Jr, MD, MBA". Retrieved 2007-07-19.
- ↑ 4.0 4.1 Saruhan-Direskeneli, G (Jul 2, 2013). "Identification of Multiple Genetic Susceptibility Loci in Takayasu Arteritis". American journal of human genetics. 93 (2): 298–305. doi:10.1016/j.ajhg.2013.05.026. PMC 3738826. PMID 23830517. Unknown parameter
|coauthors=ignored (help) - ↑ Shikino Kiyoshi, Takako Masuyama and Masatomi Ikusaka. FDG-PET of Takayasu Arteritis. JGIM 2014.
- ↑ Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M; et al. (1994). "Takayasu arteritis". Ann Intern Med. 120 (11): 919–29. PMID 7909656.
- ↑ Yoneda S, Nukada T, Tada K, Imaizumi M, Takano T (1977). "Subclavian steal in Takayasu's arteritis. A hemodynamic study by means of ultrasonic Doppler flowmetry". Stroke. 8 (2): 264–8. PMID 15335.
- ↑ Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM et al. (1990) The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33 (8):1129-34. PMID: 1975175 PMID: 1975175
- ↑ 9.0 9.1 "Takayasu Arteritis: eMedicine Pediatrics: General Medicine".
- ↑ Yamada I, Nakagawa T, Himeno Y: Takayasu arteritis: diagnosis with breath-hold contrast-enhanced three- dimensional MR angiography. J Magn Reson Imaging 2000; 11: 481
- ↑ www.fda.gov
Template:Diseases of the musculoskeletal system and connective tissue