Sumatriptan (oral): Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
|authorTag={{DB}} | |authorTag={{DB}} | ||
|aOrAn=a | |aOrAn=a | ||
|indication=migraine with or without aura in adults | |||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions= | |adverseReactions=paresthesia, warm/cold sensation, chest pain/tightness/pressure and/or heaviness, neck/throat/jaw pain/tightness/pressure, other sensations of pain/pressure/tightness/heaviness, vertigo, and malaise/fatigue | ||
|blackBoxWarningTitle=Title | |blackBoxWarningTitle=Title | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 12: | Line 13: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult====== | |fdaLIADAdult======Migraine with or without aura===== | ||
Sumatriptan tablets, USP are indicated for the acute treatment of migraine with or without aura in adults. | |||
* Dosing Information | * Dosing Information | ||
:* | :*The recommended dose of sumatriptan tablets is 25 mg, 50 mg, or 100 mg. Doses of 50 mg and 100 mg may provide a greater effect than the 25 mg dose, but doses of 100 mg may not provide a greater effect than the 50 mg dose. Higher doses may have a greater risk of adverse reactions [see Clinical Studies (14)]. | ||
If the migraine has not resolved by 2 hours after taking sumatriptan tablets, or returns after a transient improvement, a second dose may be administered at least 2 hours after the first dose. The maximum daily dose is 200 mg in a 24-hour period. | |||
Use after sumatriptan injection: If the migraine returns following an initial treatment with sumatriptan succinate injection, additional single sumatriptan tablets (up to 100 mg/day) may be given with an interval of at least 2 hours between tablet doses. | |||
The safety of treating an average of more than 4 headaches in a 30-day period has not been established. | |||
2.2 Dosing in Patients With Hepatic Impairment | |||
If treatment is deemed advisable in the presence of mild to moderate hepatic impairment, the maximum single dose should not exceed 50 mg [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. | |||
====Limitations of Use:==== | |||
Use only if a clear diagnosis of migraine headache has been established. If a patient has no response to the first migraine attack treated with sumatriptan tablets, USP, reconsider the diagnosis of migraine before sumatriptan tablets, USP are administered to treat any subsequent attacks. | |||
Sumatriptan tablets, USP are not indicated for the prevention of migraine attacks. | |||
Safety and effectiveness of sumatriptan tablets have not been established for cluster headache. | |||
=====Condition2===== | =====Condition2===== | ||
| Line 110: | Line 129: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications= | |contraindications=Sumatriptan tablets are contraindicated in patients with: | ||
Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal’s angina [see Warnings and Precautions (5.1)] | |||
Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see Warnings and Precautions (5.2)] | |||
History of stroke or transient ischemic attack (TIA) or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke [see Warnings and Precautions (5.4)] | |||
Peripheral vascular disease [see Warnings and Precautions (5.5)] | |||
Ischemic bowel disease [see Warnings and Precautions (5.5)] | |||
Uncontrolled hypertension [see Warnings and Precautions (5.8)] | |||
Recent use (i.e., within 24 hours) of ergotamine-containing medication, ergot-type medication (such as dihydroergotamine or methysergide), or another 5-hydroxytryptamine 1 (5-HT 1) agonist [see Drug Interactions (7.1, 7.3)] | |||
Concurrent administration of a monoamine oxidase (MAO)-A inhibitor or recent (within 2 weeks) use of an MAO-A inhibitor [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)] | |||
Hypersensitivity to sumatriptan (angioedema and anaphylaxis seen) [see Warnings and Precautions (5.9)] | |||
Severe hepatic impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)] | |||
|warnings=5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal’s Angina | |||
The use of sumatriptan tablets is contraindicated in patients with ischemic or vasospastic CAD. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of sumatriptan tablets. Some of these reactions occurred in patients without known CAD. Sumatriptan tablets may cause coronary artery vasospasm (Prinzmetal’s angina), even in patients without a history of CAD. | |||
Perform a cardiovascular evaluation in triptan-naive patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving sumatriptan tablets. If there is evidence of CAD or coronary artery vasospasm, sumatriptan tablets are contraindicated. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administering the first dose of sumatriptan tablets in a medically supervised setting and performing an electrocardiogram (ECG) immediately following administration of sumatriptan tablets. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of sumatriptan tablets. | |||
5.2 Arrhythmias | |||
Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT 1 agonists. Discontinue sumatriptan tablets if these disturbances occur. Sumatriptan tablets are contraindicated in patients with Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders. | |||
5.3 Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure | |||
Sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with sumatriptan tablets and are usually non-cardiac in origin. However, perform a cardiac evaluation if these patients are at high cardiac risk. The use of sumatriptan tablets is contraindicated in patients with CAD and those with Prinzmetal’s variant angina. | |||
5.4 Cerebrovascular Events | |||
Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT 1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, TIA). Discontinue sumatriptan tablets if a cerebrovascular event occurs. | |||
Before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, exclude other potentially serious neurological conditions. Sumatriptan tablets are contraindicated in patients with a history of stroke or TIA. | |||
5.5 Other Vasospasm Reactions | |||
Sumatriptan tablets may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT 1 agonist, rule out a vasospastic reaction before receiving additional sumatriptan tablets. | |||
Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT 1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1 agonists have not been clearly established. | |||
5.6 Medication Overuse Headache | |||
Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, or combination of these drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary. | |||
5.7 Serotonin Syndrome | |||
Serotonin syndrome may occur with sumatriptan tablets, particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors [see Drug Interactions (7.4)]. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms usually occurs within minutes to hours of receiving a new or a greater dose of a serotonergic medication. Discontinue sumatriptan tablets if serotonin syndrome is suspected. | |||
5.8 Increase in Blood Pressure | |||
Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients treated with 5-HT 1 agonists, including patients without a history of hypertension. Monitor blood pressure in patients treated with sumatriptan. Sumatriptan tablets are contraindicated in patients with uncontrolled hypertension. | |||
5.9 Anaphylactic/Anaphylactoid Reactions | |||
Anaphylactic/anaphylactoid reactions have occurred in patients receiving sumatriptan. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens. Sumatriptan tablets are contraindicated in patients with a history of hypersensitivity reaction to sumatriptan. | |||
5.10 Seizures | |||
Seizures have been reported following administration of sumatriptan. Some have occurred in patients with either a history of seizures or concurrent conditions predisposing to seizures. There are also reports in patients where no such predisposing factors are apparent. Sumatriptan tablets should be used with caution in patients with a history of epilepsy or conditions associated with a lowered seizure threshold. | |||
|clinicalTrials=6.1 Clinical Trials Experience | |||
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
Table 1 lists adverse reactions that occurred in placebo-controlled clinical trials in patients who took at least 1 dose of study drug. Only treatment-emergent adverse reactions that occurred at a frequency of 2% or more in any group treated with sumatriptan tablets and that occurred at a frequency greater than the placebo group are included in Table 1. | |||
[[File:Sumatriptan table 1.png|600px|thumbnail|left]] | |||
{{clear}} | |||
The incidence of adverse reactions in controlled clinical trials was not affected by gender or age of the patients. There were insufficient data to assess the impact of race on the incidence of adverse reactions. | |||
|postmarketing=The following adverse reactions have been identified during postapproval use of sumatriptan tablets, sumatriptan nasal spray, and sumatriptan injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to sumatriptan or a combination of these factors. | |||
Cardiovascular: Hypotension, palpitations. | |||
Neurological: Dystonia, tremor. | |||
|drugInteractions=* Drug | |drugInteractions=* Drug | ||
:* Description | :* Description | ||
| Line 264: | Line 222: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration= | |administration=2.1 Dosing Information | ||
The recommended dose of sumatriptan tablets is 25 mg, 50 mg, or 100 mg. Doses of 50 mg and 100 mg may provide a greater effect than the 25 mg dose, but doses of 100 mg may not provide a greater effect than the 50 mg dose. Higher doses may have a greater risk of adverse reactions [see Clinical Studies (14)]. | |||
If the migraine has not resolved by 2 hours after taking sumatriptan tablets, or returns after a transient improvement, a second dose may be administered at least 2 hours after the first dose. The maximum daily dose is 200 mg in a 24-hour period. | |||
Use after sumatriptan injection: If the migraine returns following an initial treatment with sumatriptan succinate injection, additional single sumatriptan tablets (up to 100 mg/day) may be given with an interval of at least 2 hours between tablet doses. | |||
The safety of treating an average of more than 4 headaches in a 30-day period has not been established. | |||
2.2 Dosing in Patients With Hepatic Impairment | |||
If treatment is deemed advisable in the presence of mild to moderate hepatic impairment, the maximum single dose should not exceed 50 mg [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. | |||
Close | |||
3 DOSAGE FORMS AND STRENGTHS | |||
25 mg Tablets: White to off-white, round, biconvex, uncoated tablets, debossed with “RI61” on one side and plain on the other side. | |||
50 mg Tablets: White to off-white, round, biconvex, uncoated tablets, debossed with “RI62” on one side and plain on the other side. | |||
100 mg Tablets: White to off-white, capsule-shaped, biconvex, uncoated tablets, debossed with “RB97” on one side and plain on the other side. | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
| Line 292: | Line 268: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox= | |drugBox=[[File:Sumatriptan image.png|600px|thumbnail|left]] | ||
{{clear}} | |||
|mechAction=* | |mechAction=* | ||
<!--Structure--> | <!--Structure--> | ||
|structure= | |structure= | ||
[[File:Sumatriptan structure.jpg|600px|thumbnail|left]] | |||
{{clear}} | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
| Line 316: | Line 292: | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
| | |packLabel=[[File:Sumatriptan pdp.jpg|600px|thumbnail|left]] | ||
{{clear}} | |||
[[File:Sumatriptan label.png|600px|thumbnail|left]] | |||
{{clear}} | |||
|fdaPatientInfo= | |||
[[File:Sumatriptan medication guide.png|600px|thumbnail|left]] | |||
{{clear}} | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
Revision as of 16:51, 4 December 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Sumatriptan (oral) is a {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of migraine with or without aura in adults. There is a Black Box Warning for this drug as shown here. Common adverse reactions include paresthesia, warm/cold sensation, chest pain/tightness/pressure and/or heaviness, neck/throat/jaw pain/tightness/pressure, other sensations of pain/pressure/tightness/heaviness, vertigo, and malaise/fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Migraine with or without aura
Sumatriptan tablets, USP are indicated for the acute treatment of migraine with or without aura in adults.
- Dosing Information
- The recommended dose of sumatriptan tablets is 25 mg, 50 mg, or 100 mg. Doses of 50 mg and 100 mg may provide a greater effect than the 25 mg dose, but doses of 100 mg may not provide a greater effect than the 50 mg dose. Higher doses may have a greater risk of adverse reactions [see Clinical Studies (14)].
If the migraine has not resolved by 2 hours after taking sumatriptan tablets, or returns after a transient improvement, a second dose may be administered at least 2 hours after the first dose. The maximum daily dose is 200 mg in a 24-hour period.
Use after sumatriptan injection: If the migraine returns following an initial treatment with sumatriptan succinate injection, additional single sumatriptan tablets (up to 100 mg/day) may be given with an interval of at least 2 hours between tablet doses.
The safety of treating an average of more than 4 headaches in a 30-day period has not been established.
2.2 Dosing in Patients With Hepatic Impairment
If treatment is deemed advisable in the presence of mild to moderate hepatic impairment, the maximum single dose should not exceed 50 mg [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Limitations of Use:
Use only if a clear diagnosis of migraine headache has been established. If a patient has no response to the first migraine attack treated with sumatriptan tablets, USP, reconsider the diagnosis of migraine before sumatriptan tablets, USP are administered to treat any subsequent attacks. Sumatriptan tablets, USP are not indicated for the prevention of migraine attacks. Safety and effectiveness of sumatriptan tablets have not been established for cluster headache.
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Sumatriptan (oral) in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sumatriptan (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Sumatriptan (oral) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Sumatriptan (oral) in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sumatriptan (oral) in pediatric patients.
Contraindications
Sumatriptan tablets are contraindicated in patients with:
Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal’s angina [see Warnings and Precautions (5.1)] Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see Warnings and Precautions (5.2)] History of stroke or transient ischemic attack (TIA) or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke [see Warnings and Precautions (5.4)] Peripheral vascular disease [see Warnings and Precautions (5.5)] Ischemic bowel disease [see Warnings and Precautions (5.5)] Uncontrolled hypertension [see Warnings and Precautions (5.8)] Recent use (i.e., within 24 hours) of ergotamine-containing medication, ergot-type medication (such as dihydroergotamine or methysergide), or another 5-hydroxytryptamine 1 (5-HT 1) agonist [see Drug Interactions (7.1, 7.3)] Concurrent administration of a monoamine oxidase (MAO)-A inhibitor or recent (within 2 weeks) use of an MAO-A inhibitor [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)] Hypersensitivity to sumatriptan (angioedema and anaphylaxis seen) [see Warnings and Precautions (5.9)] Severe hepatic impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal’s Angina
The use of sumatriptan tablets is contraindicated in patients with ischemic or vasospastic CAD. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of sumatriptan tablets. Some of these reactions occurred in patients without known CAD. Sumatriptan tablets may cause coronary artery vasospasm (Prinzmetal’s angina), even in patients without a history of CAD.
Perform a cardiovascular evaluation in triptan-naive patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving sumatriptan tablets. If there is evidence of CAD or coronary artery vasospasm, sumatriptan tablets are contraindicated. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administering the first dose of sumatriptan tablets in a medically supervised setting and performing an electrocardiogram (ECG) immediately following administration of sumatriptan tablets. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of sumatriptan tablets.
5.2 Arrhythmias
Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT 1 agonists. Discontinue sumatriptan tablets if these disturbances occur. Sumatriptan tablets are contraindicated in patients with Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders.
5.3 Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure
Sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with sumatriptan tablets and are usually non-cardiac in origin. However, perform a cardiac evaluation if these patients are at high cardiac risk. The use of sumatriptan tablets is contraindicated in patients with CAD and those with Prinzmetal’s variant angina.
5.4 Cerebrovascular Events
Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT 1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, TIA). Discontinue sumatriptan tablets if a cerebrovascular event occurs.
Before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, exclude other potentially serious neurological conditions. Sumatriptan tablets are contraindicated in patients with a history of stroke or TIA.
5.5 Other Vasospasm Reactions
Sumatriptan tablets may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT 1 agonist, rule out a vasospastic reaction before receiving additional sumatriptan tablets.
Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT 1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1 agonists have not been clearly established.
5.6 Medication Overuse Headache
Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, or combination of these drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
5.7 Serotonin Syndrome
Serotonin syndrome may occur with sumatriptan tablets, particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors [see Drug Interactions (7.4)]. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms usually occurs within minutes to hours of receiving a new or a greater dose of a serotonergic medication. Discontinue sumatriptan tablets if serotonin syndrome is suspected.
5.8 Increase in Blood Pressure
Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients treated with 5-HT 1 agonists, including patients without a history of hypertension. Monitor blood pressure in patients treated with sumatriptan. Sumatriptan tablets are contraindicated in patients with uncontrolled hypertension.
5.9 Anaphylactic/Anaphylactoid Reactions
Anaphylactic/anaphylactoid reactions have occurred in patients receiving sumatriptan. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens. Sumatriptan tablets are contraindicated in patients with a history of hypersensitivity reaction to sumatriptan.
5.10 Seizures
Seizures have been reported following administration of sumatriptan. Some have occurred in patients with either a history of seizures or concurrent conditions predisposing to seizures. There are also reports in patients where no such predisposing factors are apparent. Sumatriptan tablets should be used with caution in patients with a history of epilepsy or conditions associated with a lowered seizure threshold.
Adverse Reactions
Clinical Trials Experience
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Table 1 lists adverse reactions that occurred in placebo-controlled clinical trials in patients who took at least 1 dose of study drug. Only treatment-emergent adverse reactions that occurred at a frequency of 2% or more in any group treated with sumatriptan tablets and that occurred at a frequency greater than the placebo group are included in Table 1.
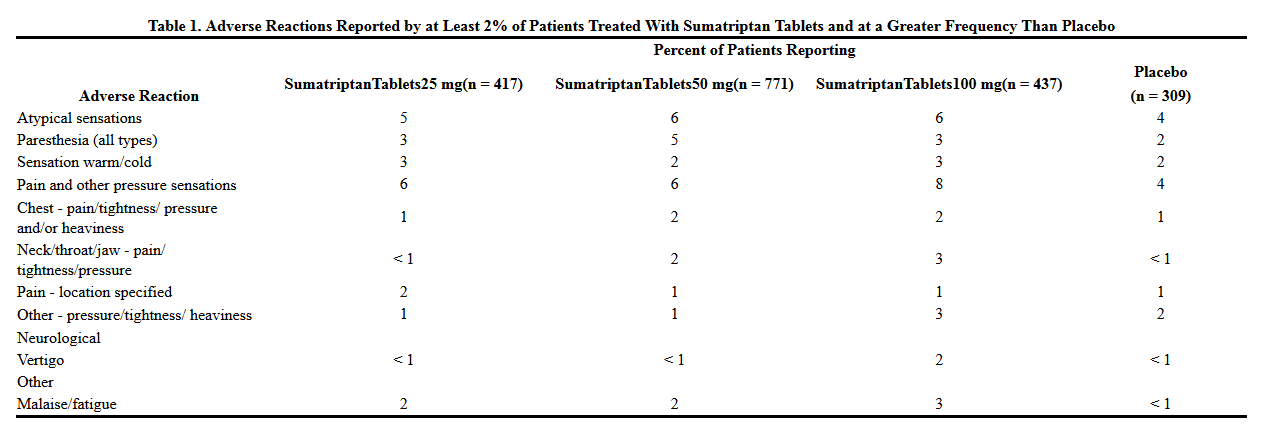
The incidence of adverse reactions in controlled clinical trials was not affected by gender or age of the patients. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of sumatriptan tablets, sumatriptan nasal spray, and sumatriptan injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to sumatriptan or a combination of these factors.
Cardiovascular: Hypotension, palpitations.
Neurological: Dystonia, tremor.
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sumatriptan (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sumatriptan (oral) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Sumatriptan (oral) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Sumatriptan (oral) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Sumatriptan (oral) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Sumatriptan (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sumatriptan (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sumatriptan (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sumatriptan (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sumatriptan (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sumatriptan (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
2.1 Dosing Information
The recommended dose of sumatriptan tablets is 25 mg, 50 mg, or 100 mg. Doses of 50 mg and 100 mg may provide a greater effect than the 25 mg dose, but doses of 100 mg may not provide a greater effect than the 50 mg dose. Higher doses may have a greater risk of adverse reactions [see Clinical Studies (14)].
If the migraine has not resolved by 2 hours after taking sumatriptan tablets, or returns after a transient improvement, a second dose may be administered at least 2 hours after the first dose. The maximum daily dose is 200 mg in a 24-hour period.
Use after sumatriptan injection: If the migraine returns following an initial treatment with sumatriptan succinate injection, additional single sumatriptan tablets (up to 100 mg/day) may be given with an interval of at least 2 hours between tablet doses.
The safety of treating an average of more than 4 headaches in a 30-day period has not been established.
2.2 Dosing in Patients With Hepatic Impairment
If treatment is deemed advisable in the presence of mild to moderate hepatic impairment, the maximum single dose should not exceed 50 mg [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. Close 3 DOSAGE FORMS AND STRENGTHS
25 mg Tablets: White to off-white, round, biconvex, uncoated tablets, debossed with “RI61” on one side and plain on the other side.
50 mg Tablets: White to off-white, round, biconvex, uncoated tablets, debossed with “RI62” on one side and plain on the other side.
100 mg Tablets: White to off-white, capsule-shaped, biconvex, uncoated tablets, debossed with “RB97” on one side and plain on the other side.
Monitoring
There is limited information regarding Monitoring of Sumatriptan (oral) in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Sumatriptan (oral) in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Sumatriptan (oral) in the drug label.
Pharmacology
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Sumatriptan (oral) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Sumatriptan (oral) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Sumatriptan (oral) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Sumatriptan (oral) in the drug label.
How Supplied
Storage
There is limited information regarding Sumatriptan (oral) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sumatriptan (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

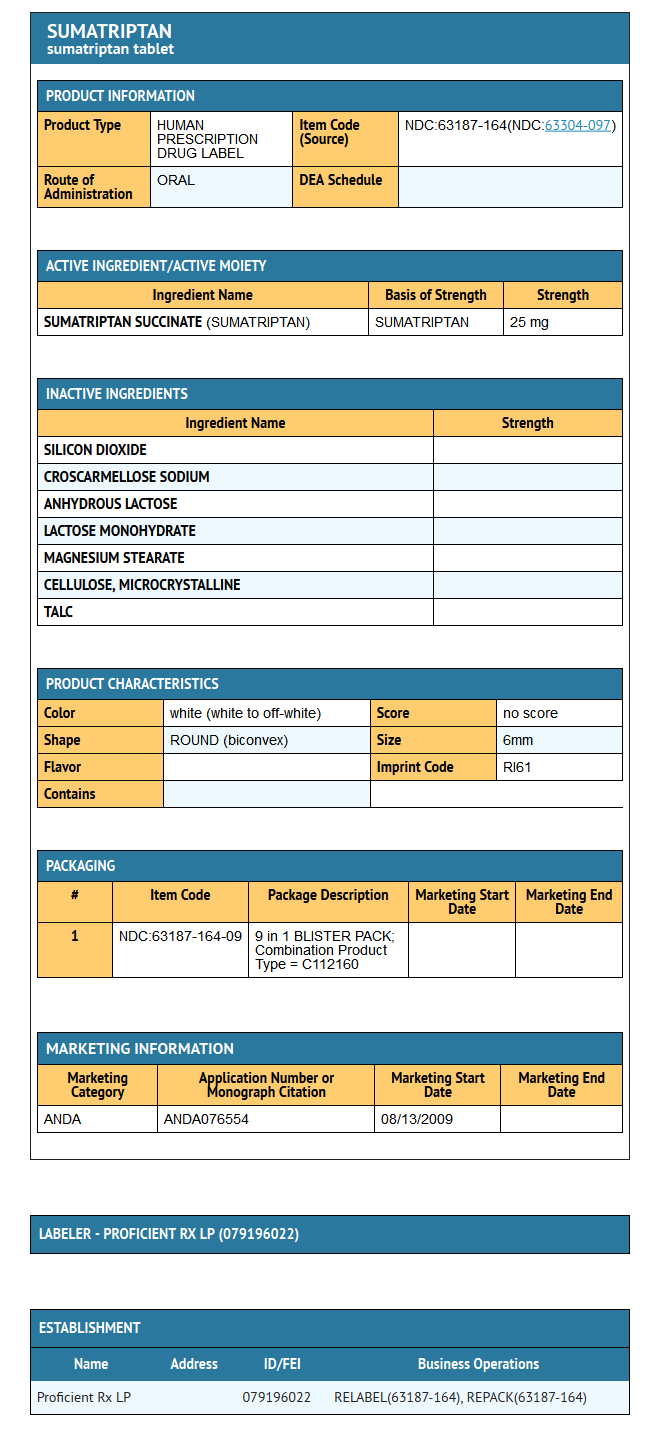
{{#ask: Label Page::Sumatriptan (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Sumatriptan (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Sumatriptan (oral)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Sumatriptan (oral) |Label Name=Sumatriptan (oral)11.png
}}
{{#subobject:
|Label Page=Sumatriptan (oral) |Label Name=Sumatriptan (oral)11.png
}}