Sultamicillin: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} +, -{{EH}} +, -{{EJ}} +, -{{Editor Help}} +, -{{Editor Join}} +)) |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
| IUPAC_name = [(2''R'')-3,3- | | Verifiedfields = changed | ||
| image = Sultamicillin. | | Watchedfields = changed | ||
| verifiedrevid = 470474605 | |||
| Verified fields = changed | |||
| verified revid = 470474605 | |||
| IUPAC_name = [(2''R'')-3,3-Dimethyl-4,4,7-trioxy-4λ<sup>6</sup>-thia-1-azabicyclo[3.2.0]heptane-2-carbonyl]oxymethyl(2''R'')-6-{[(2''S'')-2-amino-2-phenyl-acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate | |||
| image = Sultamicillin.png | |||
<!--Clinical data--> | |||
| trade name = | |||
| Drugs.com = {{drugs.com|international|sultamicillin}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S8 --> | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = | |||
| routes_of_administration = Oral | |||
<!--Pharmacokinetic data--> | |||
| bio-availability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 76497-13-7 | | CAS_number = 76497-13-7 | ||
| ATC_prefix = J01 | | ATC_prefix = J01 | ||
| ATC_suffix = CR04 | | ATC_suffix = CR04 | ||
| PubChem = | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| ChEMBL = 506110 | |||
| PubChem = 444022 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | | DrugBank = | ||
| C = 25 | H = 30 | N = 4 | O = 9 | S = 2 | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ChemSpiderID = 392048 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 65DT0ML581 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D05972 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 51770 | |||
<!--Chemical data--> | |||
| C=25 | H=30 | N=4 | O=9 | S=2 | |||
| molecular_weight = 594.659 g/mol | | molecular_weight = 594.659 g/mol | ||
| | | smiles = O=C(OCOC(=O)[C@@H]2N1C(=O)C[C@H]1S(=O)(=O)C2(C)C)[C@@H]4N5C(=O)[C@@H](NC(=O)[C@@H](c3ccccc3)N)[C@H]5SC4(C)C | ||
| InChI = 1/C25H30N4O9S2/c1-24(2)17(29-20(32)16(21(29)39-24)27-19(31)15(26)12-8-6-5-7-9-12)22(33)37-11-38-23(34)18-25(3,4)40(35,36)14-10-13(30)28(14)18/h5-9,14-18,21H,10-11,26H2,1-4H3,(H,27,31)/t14-,15-,16-,17+,18+,21-/m1/s1 | |||
| InChIKey = OPYGFNJSCUDTBT-PMLPCWDUBA | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C25H30N4O9S2/c1-24(2)17(29-20(32)16(21(29)39-24)27-19(31)15(26)12-8-6-5-7-9-12)22(33)37-11-38-23(34)18-25(3,4)40(35,36)14-10-13(30)28(14)18/h5-9,14-18,21H,10-11,26H2,1-4H3,(H,27,31)/t14-,15-,16-,17+,18+,21-/m1/s1 | |||
| | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChIKey = OPYGFNJSCUDTBT-PMLPCWDUSA-N | |||
| | |||
| | |||
| | |||
}} | }} | ||
__Notoc__ | |||
{{SI}} | {{SI}} | ||
{{CMG}} | |||
==Overview== | |||
'''Sultamicillin''' is an oral form of the antibiotic combination ([[codrug]] or mutual [[prodrug]]) [[ampicillin/sulbactam]]. It contains [[ester]]ified [[ampicillin]] and [[sulbactam]] and is marketed under a number of trade names, including '''Saltum''' from Morepen Lab and '''Unasyn''' from [[Pfizer]]. | |||
The pharmacokinetic properties of sultamicillin are improved compared to a combination of ampicillin and sulbactam. Sultamicillin increases the absorption and decreases the chances of [[diarrhea]] and [[dysentery]]. The inclusion of sulbactam extends ampicillin's spectrum of action to [[beta-lactamase]] producing strains of bacteria. Oral sulbactam with parenteral form provides a regimen of continuous sulbactam therapy throughout the treatment, resulting in better clinical results. | |||
== | ==Chemical evaluation== | ||
Sultamicillin is a mutual prodrug of ampicillin and sulbactam. Ampicillin, a [[semi-synthetic]] orally active broad spectrum antibiotic, is linked via a [[methylene group]] with a beta-lactamase inhibitor. Sultamicillin is chemically oxymethyl penicillinate sulfone ester of ampicillin. | |||
==Mechanism of action== | |||
After absorption, sultamicillin releases ampicillin and sulbactam into the system, so all the antibacterial efficacy of sultamicillin is due to ampicillin and sulbactam. Ampicillin exerts antibacterial activity against sensitive organisms by inhibiting biosynthesis of cell wall [[mucopeptide]] where as sulbactam irreversibly inhibits most important beta-lactamases that occur in resistant strains. | |||
[[ | ==Indications== | ||
[[Indication (medicine)|Indication]]s for sultamicillin include: | |||
* Skin and soft tissue infections - [[furuncle]]s, [[carbuncle]]s, [[cellulitis]], [[paronychia]], [[impetigo contagiosa]], [[diabetic foot ulcer]]s and abscesses caused by ''[[Staphylococcus aureus]]'' and ''[[Streptococcus pyogenes]]''. | |||
* [[Upper respiratory tract infection]]s - [[pharyngitis]] and [[tonsillitis]] caused by ''[[S. pyogenes]]'' and ''S. aureus''. Acute and chronic sinusitis caused by ''S. aureus'', ''[[S. pneumoniae]]'', ''[[H. influenzae]]'' and ''S. progenies''. [[Otis media]], particularly suppurative otis media, with or without [[mastoiditis antrum]]. | |||
* [[Lower respiratory tract infection]]s - [[bacterial pneumonias]], [[bronchitis]], [[bronchiestasis]] caused by ''S. pneumoniae'', ''H. influenzae'', ''Staphylococcus aureus'' and ''S. progenies''. Acute exacerbations of [[COPD]]. | |||
* [[Urinary tract infection]]s - [[pyelonephritis]], [[cystitis]] caused by ''[[Escherichia coli]]'', ''[[Proteus mirabilis]]'', ''[[Klebsiella]]'', ''[[Enterobacter]]'' and ''Staphylococcus aureus''. | |||
* Surgical infections - prophylaxis and treatment of surgical site infections, peri-operative prophylaxis in orthopaedic and cardiovascular surgery. | |||
* Gynecological infections - Caused by beta-lactamase producing strains of ''E. coli'' and ''[[Bacteroides]]'' sp. (including ''[[B. fragilis]]''). | |||
* Infections of the [[gastrointestinal tract]] - Bacterial [[esophagitis]], treatment of ''[[H. pylori]]'' infections as a part of MDT in ulcer management. | |||
==References== | |||
{{nofootnotes|date=September 2014}} | |||
* {{cite journal | doi =10.2174/1389557043487547 | pmid = 14754446| year = 2004| author1 = Singh| first1 = G.S.| title = Beta-lactams in the new millennium. Part-II: Cephems, oxacephems, penams and sulbactam| journal = Mini Reviews in Medicinal Chemistry| volume = 4| issue = 1| pages = 93–109}} | |||
{{Cell wall disruptive antibiotics}} | |||
[[ | [[Category:Drug]] | ||
[[Category:Beta-lactam antibiotics]] | |||
Revision as of 14:09, 9 April 2015
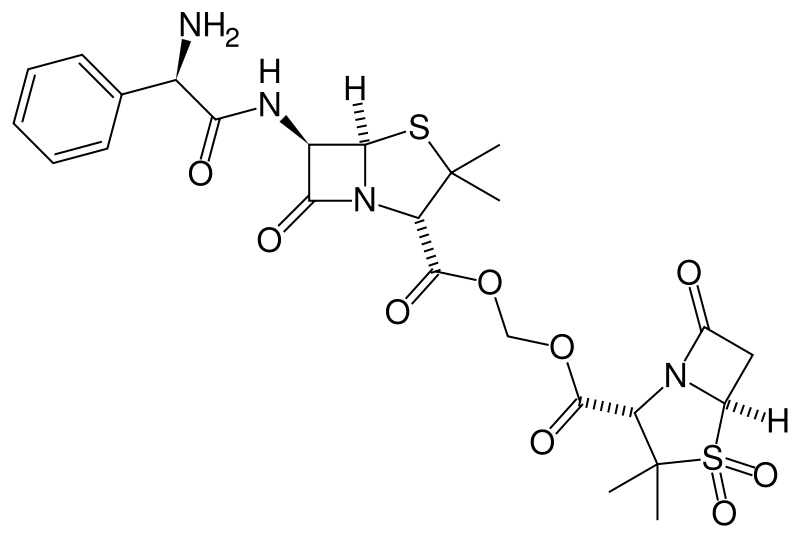 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C25H30N4O9S2 |
| Molar mass | 594.659 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Sultamicillin |
|
Articles |
|---|
|
Most recent articles on Sultamicillin Most cited articles on Sultamicillin |
|
Media |
|
Powerpoint slides on Sultamicillin |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Sultamicillin at Clinical Trials.gov Trial results on Sultamicillin Clinical Trials on Sultamicillin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Sultamicillin NICE Guidance on Sultamicillin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Sultamicillin Discussion groups on Sultamicillin Patient Handouts on Sultamicillin Directions to Hospitals Treating Sultamicillin Risk calculators and risk factors for Sultamicillin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Sultamicillin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Sultamicillin is an oral form of the antibiotic combination (codrug or mutual prodrug) ampicillin/sulbactam. It contains esterified ampicillin and sulbactam and is marketed under a number of trade names, including Saltum from Morepen Lab and Unasyn from Pfizer.
The pharmacokinetic properties of sultamicillin are improved compared to a combination of ampicillin and sulbactam. Sultamicillin increases the absorption and decreases the chances of diarrhea and dysentery. The inclusion of sulbactam extends ampicillin's spectrum of action to beta-lactamase producing strains of bacteria. Oral sulbactam with parenteral form provides a regimen of continuous sulbactam therapy throughout the treatment, resulting in better clinical results.
Chemical evaluation
Sultamicillin is a mutual prodrug of ampicillin and sulbactam. Ampicillin, a semi-synthetic orally active broad spectrum antibiotic, is linked via a methylene group with a beta-lactamase inhibitor. Sultamicillin is chemically oxymethyl penicillinate sulfone ester of ampicillin.
Mechanism of action
After absorption, sultamicillin releases ampicillin and sulbactam into the system, so all the antibacterial efficacy of sultamicillin is due to ampicillin and sulbactam. Ampicillin exerts antibacterial activity against sensitive organisms by inhibiting biosynthesis of cell wall mucopeptide where as sulbactam irreversibly inhibits most important beta-lactamases that occur in resistant strains.
Indications
Indications for sultamicillin include:
- Skin and soft tissue infections - furuncles, carbuncles, cellulitis, paronychia, impetigo contagiosa, diabetic foot ulcers and abscesses caused by Staphylococcus aureus and Streptococcus pyogenes.
- Upper respiratory tract infections - pharyngitis and tonsillitis caused by S. pyogenes and S. aureus. Acute and chronic sinusitis caused by S. aureus, S. pneumoniae, H. influenzae and S. progenies. Otis media, particularly suppurative otis media, with or without mastoiditis antrum.
- Lower respiratory tract infections - bacterial pneumonias, bronchitis, bronchiestasis caused by S. pneumoniae, H. influenzae, Staphylococcus aureus and S. progenies. Acute exacerbations of COPD.
- Urinary tract infections - pyelonephritis, cystitis caused by Escherichia coli, Proteus mirabilis, Klebsiella, Enterobacter and Staphylococcus aureus.
- Surgical infections - prophylaxis and treatment of surgical site infections, peri-operative prophylaxis in orthopaedic and cardiovascular surgery.
- Gynecological infections - Caused by beta-lactamase producing strains of E. coli and Bacteroides sp. (including B. fragilis).
- Infections of the gastrointestinal tract - Bacterial esophagitis, treatment of H. pylori infections as a part of MDT in ulcer management.
References
- Singh, G.S. (2004). "Beta-lactams in the new millennium. Part-II: Cephems, oxacephems, penams and sulbactam". Mini Reviews in Medicinal Chemistry. 4 (1): 93–109. doi:10.2174/1389557043487547. PMID 14754446.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drug
- Beta-lactam antibiotics