Stomach cancer pathophysiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Omer Kamal, M.D.[2], Parminder Dhingra, M.D. [3], Mohammed Abdelwahed M.D[4]
|
Stomach cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Stomach cancer pathophysiology On the Web |
|
American Roentgen Ray Society Images of Stomach cancer pathophysiology |
|
Risk calculators and risk factors for Stomach cancer pathophysiology |
Overview
Gastric cancer may occur secondary to a variety of causes including H. pylori and gastric cancer have strong correlation. This is related to nitric oxide accumulation produced by inflammatory cells responding to H. pylori infection. The pathophysiology of stomach cancer depends upon the histologic subtype. K-ras mutations is found in invasive cancers and intestinal metaplasia. Inactivation of p53 in gastric epithelial cells reduce their ability to undergo apoptosis. DNA methylation of gene promoters can silence the expression of CDH1. Beta-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Diffuse gastric carcinomas do not have a precancerous lesion. They are highly metastatic with a poorer prognosis than intestinal cancers. When the entire stomach wall is infiltrated, it results in a rigid thickened stomach wall called linitis plastica. There are many diseases associated with gastric cancer such as, hereditary diffuse gastric cancer, gastric adenocarcinoma, proximal polyposis of the stomach, Lynch syndrome, familial adenomatous polyposis, Li-Fraumeni syndrome, Peutz Jeghers syndrome, juvenile polyposis, hereditary breast and ovarian cancer syndrome and Cowden's syndrome. There are five gross pathological types of gastric cancer; superficical, ulcerative, infiltrative ulcerative, diffuse infiltrative, and unclassified. There are two major histological classifications for gastric cancer including Japanese classification and WHO classification. The main two types are intestinal type adenocarcinoma and diffuse type adenocarcinoma.
Physiology of gastric acid secretion
- The stomach consists of two functional areas; oxyntic and pyloric glands. The oxyntic area contains parietal cells that produce gastric acid.
- Parietal cells are filled with secretory vesicles that coalesce with stimulation to form channels that drain to the apical lumen.[1]
- The secretory membrane contains hydrogen-potassium-ATPase acid-secreting pump. While stimulated hydrogen-potassium exchange occur. The collected hydrogen unifies with chloride forming hydrochloric acid.[2]
- The antrum contains pyloric glands that secrete gastrin and somatostatin.
- Gastrin enhances gastric acid secretions from parietal cells by increasing synthesis of histamine.[3]
- Somatostatin: The secretion of somatostatin is increased by gastric acid and gastrin level.[4]
Pathophysiology of gastric cancer
The pathophysiology of gastric cancer is based on various factors leading to decreased apoptosis, increased proliferation and abnormal differentiation of gastric epithelial cells. The following etiological factors contribute to the development of gastric cancer:[5]
- Helicobacter pylori infection leading to activation and dysregulation of three signaling pathways, involving three major components:[6]
- Nuclear factor-κB
- Wnt/β-catenin
- Proliferation/stem cell
- Dietary habits involving high consumption of starch, decreased consumption of high quality protein, fresh fruits and vegetables. These diets favor acid-catalyzed nitrosation in the stomach and leads to mechanical damage to the gastric mucosa.
- Family history of hereditary conditions which may lead to an increased risk of gastric cancer for example, Li-Fraumeni syndrome and hereditary non-polyposis colon cancer.[7]
Pathogenesis of intestinal type gastric cancer
Molecular effect of H.pylori:
- There is a strong correlation between Helicobacter pylori and gastric cancer incidence.[8]
- The main cause of this correlation is related to nitric oxides accumulation produced by inflammatory cells responding to H. pylori infection.[9]
- Nitric oxides may induce abnormalities in the DNA of epithelial cells.
- Incidences of gastric cancer has been known to decrease with eradication of H. pylori infection.[10]
- The exact pathway for oncogenesis is not known but many trials support the adenoma-carcinoma sequence.
- The intestinal type of gastric adenocarcinoma is the most common type of gastric cancer. The development of intestinal type gastric carcinoma progresses from chronic superficial (non atrophic) gastritis, to chronic atrophic gastritis to intestinal metaplasia, followed by dysplasia and finally, gastric adenocarcinoma.[11]
- H. pylori hosts a virulence factor called cytotoxin-associated gene A (cag A) which leads to induction of growth factor receptors, increased proliferation, inhibition of apoptosis, and promotion of invasion and angiogenesis.[12]
- H. pylori also leads to production of reactive oxygen and nitrogen species and leads to suppression of the host antioxidant defense mechanisms, causing oxidative DNA damage.[13]
Beta-catenin/Wnt signaling
- Beta-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer.[14]
- Beta-catenin is a part of Wnt signaling pathway which regulates coordination of events such as intercellular adhesion junctions, migration, proliferation, and differentiation.
- Beta-catenin is normally bound to protein complexes in the cell membrane that are involved in normal intercellular adhesions.
- APC gene protein prevents the accumulation of beta-catenin. APC mutations lead to loss of regulation of beta-catenin which leads to proliferation, angiogenesis, tumor invasion, and metastasis of cells.[15]
{{#ev:youtube|oweNT288BXo}}
Pathogenesis of diffuse-type gastric cancer
- Diffuse gastric carcinomas do not have a precancerous lesion.
- They are highly metastatic with a poorer prognosis than intestinal cancers. When the entire stomach wall is infiltrated, it results in a rigid thickened stomach wall called linitis plastic.[16]
- Intracellular mucin pushes the nucleus giving the histological figure of signet ring carcinoma.
- The E-cadherin gene (CDH1) encodes a transmembrane cellular adhesion protein. Its cytoplasmic tail interacts with catenins making the adhesion.
- Somatic mutations in the CDH1 gene by hypermethylation, mutation, and loss of heterozygosity are identified in 40 to 83 percent of sporadic diffuse-type gastric cancers.[17]
- Prostate stem cell antigen gene is also involved in regulating gastric epithelial cell proliferation.[18]
Apoptosis pathway
Neutrophil activation
- H. pylori infection results in the migration of neutrophils to the site of infection and adhesion to the surface epithelium.
- The neutrophils produce nitric oxide synthase which damage DNA.
- CD11a/CD18- and CD11b/CD18-neutrophils interact with intercellular adhesion molecule-1 (ICAM-1).[19]
- Epithelial cells respond by signaling pathways leading to apoptosis, proliferation, differentiation, and autophagy.
Apoptotic pathways
- Apoptosis occurs as a protective mechanism to prevent replication of mutated DNA which leads to atrophy of epithelium so called atrophic gastritis which returns to normal following eradication therapy.[20]
- H. pylori enhances expression of the Fas receptor on gastric epithelial cells and may mediate apoptosis through signaling mechanisms related to the Fas death receptor.
- Another trial supported that the source of tumorigenesis is from bone marrow-derived cells that differentiate into gastric epithelial cells in the presence of H. pylori.[21]
{{#ev:youtube|SyvOPXeg4ig}}
| Helicobacter pylori infection | |||||||||||||||||||||||||||||||||||||
| Inflammatory response secretes IL-8 ,IL-1b | Production of alkaline ammonia | Production of urease bacterial phospholipase A | |||||||||||||||||||||||||||||||||||
| Infux of neutophils and macrophages release of lysosomal enzymes leukotrienes (LT)and reactive oxygen | inhibition of D-cells leads to inappropriate release of somatostatin and hypergastrinemia | Production of urease ,phospholipase A and C release toxic metabolities | |||||||||||||||||||||||||||||||||||
| Mucosal injury | |||||||||||||||||||||||||||||||||||||
Genetics
Mutations of the following genes may lead to the development of gastric cancer:
Oncogenes
- K-ras mutations are found in invasive cancers and intestinal metaplasia.[22]
- C-met receptor has a high affinity for hepatocyte growth factor (HGF). Mutations leading to increased expression of c-met are known to be associated with some types of gastric cancer ( for example intestinal-type gastric cancer). Effector protein CagA made by H.pylori modulates c-met receptor signal transduction pathways.[23][24]
Tumor suppressor genes
- Almost 50% of gastric cancers have alterations in genes TP53, TP73, APC, TFF, DCC, LOH, and FHIT.[25]
- Inactivation of p53 in gastric epithelial cells reduce their ability to undergo apoptosis.[26]
- Abnormalities are found in intestinal-type, intestinal metaplasia and dysplasia, and H. pylori-associated chronic gastritis.[27]
- Mutations in the APC gene are found in intestinal-type gastric cancers. APC mutations alternate the Wnt/catenin signaling pathway.[28]
- The trefoil factor family (TFF) is normally expressed in the gastroduodenal mucosa. Loss of TFF1 expression has been observed in gastric carcinomas.[29]
Cell cycle regulatory molecules
- Cyclin E overexpression is found in gastric carcinomas.[30]
- Cyclin E and Cyclin-dependent kinase inhibitor 1-B are cell cycle regulators.[31]
Epigenetic events
- DNA methylation of gene promoters can silence the expression of CDH1.[32]
- Hypermethylation of the Reprimo gene has been found in the plasma of patients with gastric cancer.
{{#ev:youtube|_aAhcNjmvhc}}
Associated Disorders
Familial predisposition
- Although most gastric cancers are sporadic, 10 percent of cases are familial.
Hereditary diffuse gastric cancer[33]
- CDH1 gene encodes the cell adhesion protein E-cadherin, mutations in this gene have been identified in hereditary diffuse gastric cancer.
- It is inherited as an autosomal dominant trait with high penetrance.[34]
- Promoter hypermethylation, mutation, and loss of heterozygosity result is loss of expression of the cell adhesion molecule E-cadherin.
- The asymptomatic carriers of a pathogenic CDH1 gene mutation may require prophylactic gastrectomy.
- Risk for gastric cancer for CDH1 gene mutation carriers is 70 percent in men and 56 percent in women.[35]
- Women in these affected families are also at high risk of developing breast cancer. The cumulative risk of breast cancer at 80 years of age for CDH1 mutation carriers is 42 percent.
Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS)
- GAPPS is an autosomal dominant fundic gland polyposis that is restricted to the proximal stomach, with no evidence of duodenal or colorectal polyposis or other hereditary gastrointestinal cancer syndrome.[36]
Familial intestinal gastric cancer (FIGC)
- FIGC should be considered a potential diagnosis when histopathological reports denote intestinal-type gastric cancers that segregate within families without gastric polyposis.[37]
Other hereditary cancer syndromes:[37]
- Lynch syndrome (hereditary nonpolyposis colorectal cancer)
- Familial adenomatous polyposis (FAP)
- Li-Fraumeni syndrome
- Peutz Jeghers syndrome
- juvenile polyposis
- Hereditary breast and ovarian cancer syndrome
- Cowden's syndrome
Gross Pathology
The gross pathological findings in gastric cancer may be classified into the following types based on appearance of the tumor:
| Type | Description |
|---|---|
| Type 0 | (superficial) typical of T1 tumors |
| Type 1 | (mass) polypoid tumors sharply demarcated from the
surrounding mucosa |
| Type 2 | (Ulcerative) ulcerated tumors with raised margins
surrounded by a thickened gastric wall with clear margins |
| Type 3 | (Infiltrative ulcerative) ulcerated tumors with raised margins,
surrounded by a thickened gastric wall without clear margins |
| Type 4 | (Diffuse infiltrative)
Tumors without marked ulceration or raised margins, the gastric wall is thickened and indurated and the margin is unclear |
| Type 5 | (Unclassifiable)
Tumors that cannot be classified into any of the above types |
Video Showing Growth Pathology Of Gastric Cancer
{{#ev:youtube|ih-npVIJA6U}}

Histopathology
- Gastric adenocarcinoma is a malignant epithelial tumor, originating from glandular epithelium of the gastric mucosa. It invades the gastric wall, infiltrating the muscularis mucosae, the submucosa and hence the muscularis propria. Histologically, there are two major types of gastric cancer (Lauren classification): intestinal type and diffuse type.
- Intestinal type adenocarcinoma:
- Tumor cells describe irregular tubular structures, harboring pluristratification, multiple lumens, and reduced stroma. It induces intestinal metaplasia in neighboring mucosa.
- Depending on glandular architecture, cellular pleomorphism and mucosecretion, adenocarcinoma may present 3 degrees of differentiation: well, moderate and poorly differentiated.
- Diffuse type adenocarcinoma:
- Tumor cells are discohesive and secrete mucus which is delivered in the interstitium producing large pools of mucus/colloid. It is poorly differentiated. If the mucus remains inside the tumor cell, it pushes the nucleus at the periphery giving a signet ring cell appearance.
- Intestinal type adenocarcinoma:
World Health Organization histological classification of gastric tumors:
| Types | Histological features |
|---|---|
| Epithelial tumors |
|
| Non-epithelial tumors |
|
| Malignant lymphomas |
Japanese histological classification of gastric tumors:

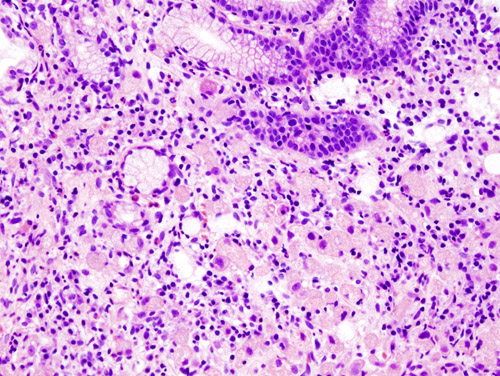
Video shows microscopic pathology of gastric cancer
{{#ev:youtube|lRvq1fEW8sY}}
{{#ev:youtube|lWeECaiEfSs}}
References
- ↑ Yao X, Forte JG (2003). "Cell biology of acid secretion by the parietal cell". Annu Rev Physiol. 65: 103–31. doi:10.1146/annurev.physiol.65.072302.114200. PMID 12500969.
- ↑ Geibel JP (2005). "Role of potassium in acid secretion". World J Gastroenterol. 11 (34): 5259–65. PMC 4622792. PMID 16149129.
- ↑ Kidd M, Modlin IM, Tang LH (1998). "Gastrin and the enterochromaffin-like cell: an acid update". Dig Surg. 15 (3): 209–17. PMID 9845587.
- ↑ Shulkes A, Read M (1991). "Regulation of somatostatin secretion by gastrin- and acid-dependent mechanisms". Endocrinology. 129 (5): 2329–34. doi:10.1210/endo-129-5-2329. PMID 1682134.
- ↑ Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y, Cheng LL, Lee J, Rha SY, Chung HC, Ganesan K, So J, Soo KC, Lim D, Chan WH, Wong WK, Bowtell D, Yeoh KG, Grabsch H, Boussioutas A, Tan P (2009). "Oncogenic pathway combinations predict clinical prognosis in gastric cancer". PLoS Genet. 5 (10): e1000676. doi:10.1371/journal.pgen.1000676. PMC 2748685. PMID 19798449.
- ↑ Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y, Cheng LL, Lee J, Rha SY, Chung HC, Ganesan K, So J, Soo KC, Lim D, Chan WH, Wong WK, Bowtell D, Yeoh KG, Grabsch H, Boussioutas A, Tan P (2009). "Oncogenic pathway combinations predict clinical prognosis in gastric cancer". PLoS Genet. 5 (10): e1000676. doi:10.1371/journal.pgen.1000676. PMC 2748685. PMID 19798449.
- ↑ Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K (2000). "Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland". N. Engl. J. Med. 343 (2): 78–85. doi:10.1056/NEJM200007133430201. PMID 10891514.
- ↑ Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ (1999). "Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis". Am J Gastroenterol. 94 (9): 2373–9. doi:10.1111/j.1572-0241.1999.01360.x. PMID 10483994.
- ↑ Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B; et al. (1996). "Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants". Cancer Res. 56 (14): 3238–43. PMID 8764115.
- ↑ Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC; et al. (2005). "Long term follow up of patients treated for Helicobacter pylori infection". Gut. 54 (11): 1536–40. doi:10.1136/gut.2005.072009. PMC 1462952. PMID 15985559.
- ↑ Correa P (1988). "A human model of gastric carcinogenesis". Cancer Res. 48 (13): 3554–60. PMID 3288329.
- ↑ Hatakeyama M (2004). "Oncogenic mechanisms of the Helicobacter pylori CagA protein". Nat. Rev. Cancer. 4 (9): 688–94. doi:10.1038/nrc1433. PMID 15343275.
- ↑ Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T (2012). "Roles of oxidative stress in stomach disorders". J Clin Biochem Nutr. 50 (1): 35–9. doi:10.3164/jcbn.11-115SR. PMC 3246180. PMID 22247598.
- ↑ Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C; et al. (2002). "beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer". Cancer Res. 62 (12): 3503–6. PMID 12067995.
- ↑ Lowy AM, Clements WM, Bishop J, Kong L, Bonney T, Sisco K; et al. (2006). "beta-Catenin/Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer". Cancer Res. 66 (9): 4734–41. doi:10.1158/0008-5472.CAN-05-4268. PMID 16651426.
- ↑ Graziano F, Humar B, Guilford P (2003). "The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice". Ann Oncol. 14 (12): 1705–13. PMID 14630673.
- ↑ Ramos-de la Medina A, More H, Medina-Franco H, Humar B, Gamboa A, Ortiz LJ; et al. (2006). "Single nucleotide polymorphisms (SNPs) at CDH1 promoter region in familial gastric cancer". Rev Esp Enferm Dig. 98 (1): 36–41. PMID 16555931.
- ↑ Study Group of Millennium Genome Project for Cancer. Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T; et al. (2008). "Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer". Nat Genet. 40 (6): 730–40. doi:10.1038/ng.152. PMID 18488030.
- ↑ Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M; et al. (2001). "Helicobacter pylori infection and the development of gastric cancer". N Engl J Med. 345 (11): 784–9. doi:10.1056/NEJMoa001999. PMID 11556297.
- ↑ Xia HH, Talley NJ (2001). "Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis". Am J Gastroenterol. 96 (1): 16–26. doi:10.1111/j.1572-0241.2001.03447.x. PMID 11197247.
- ↑ Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H; et al. (2004). "Gastric cancer originating from bone marrow-derived cells". Science. 306 (5701): 1568–71. doi:10.1126/science.1099513. PMID 15567866.
- ↑ Yasui W, Oue N, Kuniyasu H, Ito R, Tahara E, Yokozaki H (2001). "Molecular diagnosis of gastric cancer: present and future". Gastric Cancer. 4 (3): 113–21. doi:10.1007/s101200100001. PMID 11760076.
- ↑ Smith MG, Hold GL, Tahara E, El-Omar EM (2006). "Cellular and molecular aspects of gastric cancer". World J Gastroenterol. 12 (19): 2979–90. PMC 4124370. PMID 16718776.
- ↑ Inoue T, Kataoka H, Goto K, Nagaike K, Igami K, Naka D, Kitamura N, Miyazawa K (2004). "Activation of c-Met (hepatocyte growth factor receptor) in human gastric cancer tissue". Cancer Sci. 95 (10): 803–8. PMID 15504247.
- ↑ Ushiku T, Chong JM, Uozaki H, Hino R, Chang MS, Sudo M; et al. (2007). "p73 gene promoter methylation in Epstein-Barr virus-associated gastric carcinoma". Int J Cancer. 120 (1): 60–6. doi:10.1002/ijc.22275. PMID 17058198.
- ↑ Ashktorab H, Ahmed A, Littleton G, Wang XW, Allen CR, Tackey R; et al. (2003). "p53 and p14 increase sensitivity of gastric cells to H. pylori-induced apoptosis". Dig Dis Sci. 48 (7): 1284–91. PMID 12870784.
- ↑ Kodama M, Murakami K, Okimoto T, Sato R, Watanabe K, Fujioka T (2007). "Expression of mutant type-p53 products in H pylori-associated chronic gastritis". World J Gastroenterol. 13 (10): 1541–6. PMC 4146896. PMID 17461446.
- ↑ Nakatsuru S, Yanagisawa A, Furukawa Y, Ichii S, Kato Y, Nakamura Y; et al. (1993). "Somatic mutations of the APC gene in precancerous lesion of the stomach". Hum Mol Genet. 2 (9): 1463–5. PMID 8242071.
- ↑ Leung WK, Yu J, Chan FK, To KF, Chan MW, Ebert MP; et al. (2002). "Expression of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas, intestinal metaplasia, and non-neoplastic gastric tissues". J Pathol. 197 (5): 582–8. doi:10.1002/path.1147. PMID 12210076.
- ↑ Bani-Hani KE, Almasri NM, Khader YS, Sheyab FM, Karam HN (2005). "Combined evaluation of expressions of cyclin E and p53 proteins as prognostic factors for patients with gastric cancer". Clin Cancer Res. 11 (4): 1447–53. doi:10.1158/1078-0432.CCR-04-1730. PMID 15746045.
- ↑ Takano Y, Kato Y, van Diest PJ, Masuda M, Mitomi H, Okayasu I (2000). "Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases". Am J Pathol. 156 (2): 585–94. doi:10.1016/S0002-9440(10)64763-3. PMC 1850035. PMID 10666388.
- ↑ Yasui W, Sentani K, Motoshita J, Nakayama H (2006). "Molecular pathobiology of gastric cancer". Scand J Surg. 95 (4): 225–31. doi:10.1177/145749690609500403. PMID 17249269.
- ↑ Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H; et al. (2015). "Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond". JAMA Oncol. 1 (1): 23–32. doi:10.1001/jamaoncol.2014.168. PMID 26182300.
- ↑ van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N; et al. (2015). "Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers". J Med Genet. 52 (6): 361–74. doi:10.1136/jmedgenet-2015-103094. PMC 4453626. PMID 25979631.
- ↑ van der Post RS, Vogelaar IP, Manders P, van der Kolk LE, Cats A, van Hest LP; et al. (2015). "Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1". Gastroenterology. 149 (4): 897–906.e19. doi:10.1053/j.gastro.2015.06.003. PMID 26072394.
- ↑ Brosens LA, Giardiello FM, Offerhaus GJ, Montgomery EA (2016). "Syndromic Gastric Polyps: At the Crossroads of Genetic and Environmental Cancer Predisposition". Adv Exp Med Biol. 908: 347–69. doi:10.1007/978-3-319-41388-4_17. PMID 27573780.
- ↑ 37.0 37.1 Choi YJ, Kim N (2016). "Gastric cancer and family history". Korean J Intern Med. 31 (6): 1042–1053. doi:10.3904/kjim.2016.147. PMC 5094936. PMID 27809451.