Spironolactone and hydrochlorothiazide (patient information): Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 66: | Line 66: | ||
* Spironolactone (ALDACTONE®), an aldosterone antagonist, is 17-hydroxy-7α-mercapto-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone acetate and has the following structural formula: | * Spironolactone (ALDACTONE®), an aldosterone antagonist, is 17-hydroxy-7α-mercapto-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone acetate and has the following structural formula: | ||
[[File: | [[File:spirinolactone 01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
* Spironolactone is practically insoluble in water, soluble in alcohol, and freely soluble in benzene and in chloroform. | * Spironolactone is practically insoluble in water, soluble in alcohol, and freely soluble in benzene and in chloroform. | ||
| Line 79: | Line 79: | ||
|PK=* Spironolactone is rapidly and extensively metabolized. Sulfur-containing products are the predominant metabolites and are thought to be primarily responsible, together with spironolactone, for the therapeutic effects of the drug. The following pharmacokinetic data were obtained from 12 healthy volunteers following the administration of 100 mg of spironolactone (ALDACTONE film-coated tablets) daily for 15 days. On the 15th day, spironolactone was given immediately after a low fat breakfast and blood was drawn thereafter. | |PK=* Spironolactone is rapidly and extensively metabolized. Sulfur-containing products are the predominant metabolites and are thought to be primarily responsible, together with spironolactone, for the therapeutic effects of the drug. The following pharmacokinetic data were obtained from 12 healthy volunteers following the administration of 100 mg of spironolactone (ALDACTONE film-coated tablets) daily for 15 days. On the 15th day, spironolactone was given immediately after a low fat breakfast and blood was drawn thereafter. | ||
[[File: | [[File:Pharmacokinetic.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
Revision as of 17:09, 11 December 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Spironolactone and hydrochlorothiazide (patient information) is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Indications and Dosing
- Ascites - Cirrhosis of liver: maintenance, 100 mg each of spironolactone/hydrochlorothiazide ORALLY per day (range, 25 to 200 mg/day of each component) in single or divided doses, titrated based on clinical response.
- Congestive heart failure - Edema: maintenance, 100 mg each of spironolactone/hydrochlorothiazide ORALLY per day (range, 25 to 200 mg/day of each component) in single or divided doses, titrated based on clinical response.
- Edema - Nephrotic syndrome: maintenance, 100 mg each of spironolactone/hydrochlorothiazide ORALLY per day (range, 25 to 200 mg/day of each component) in single or divided doses, titrated based on clinical response.
- Hypertension: 50 to 100 mg each of spironolactone/hydrochlorothiazide ORALLY per day in single or divided doses, titrated based on clinical response.
- Hypokalemia, Diuretic-induced: 50 to 100 mg each of spironolactone/hydrochlorothiazide ORALLY per day in single or divided doses, titrated based on clinical response.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Spironolactone and hydrochlorothiazide (patient information) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- Safety and efficacy not established in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Spironolactone and hydrochlorothiazide (patient information) in pediatric patients.
Contraindications
ALDACTAZIDE is contraindicated in patients with anuria, acute renal insufficiency, significant impairment of renal excretory function, hypercalcemia, hyperkalemia, Addison's disease, and in patients who are allergic to thiazide diuretics or to other sulfonamide-derived drugs. ALDACTAZIDE may also be contraindicated in acute or severe hepatic failure.
Warnings
- Potassium supplementation, either in the form of medication or as a diet rich in potassium, should not ordinarily be given in association with ALDACTAZIDE therapy. Excessive potassium intake may cause hyperkalemia in patients receiving ALDACTAZIDE .
- Concomitant administration of ALDACTAZIDE with the following drugs or potassium sources may lead to severe hyperkalemia:
- Other potassium-sparing diuretics
- ACE inhibitors
- Angiotensin II receptor antagonists
- Aldosterone blockers
- Non-steroidal anti-inflammatory drugs (NSAIDs), e.g., indomethacin
- Heparin and low molecular weight heparin
- Other drugs or conditions known to cause hyperkalemia
- Potassium supplements
- Diet rich in potassium
- Salt substitutes containing potassium
- ALDACTAZIDE should not be administered concurrently with other potassium-sparing diuretics. Spironolactone, when used with ACE inhibitors or indomethacin, even in the presence of a diuretic, has been associated with severe hyperkalemia. Extreme caution should be exercised when ALDACTAZIDE is given concomitantly with these drugs.
- ALDACTAZIDE should be used with caution in patients with impaired hepatic function because minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
- Lithium generally should not be given with diuretics.
- Thiazides should be used with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
- Thiazides may add to or potentiate the action of other antihypertensive drugs.
- Sensitivity reactions to thiazides may occur in patients with or without a history of allergy or bronchial asthma.
- Sulfonamide derivatives, including thiazides, have been reported to exacerbate or activate systemic lupus erythematosus.
Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Spironolactone and hydrochlorothiazide (patient information) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Spironolactone and hydrochlorothiazide (patient information) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Spironolactone and hydrochlorothiazide (patient information) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Spironolactone and hydrochlorothiazide (patient information) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Spironolactone and hydrochlorothiazide (patient information) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Spironolactone and hydrochlorothiazide (patient information) in geriatric settings.
Gender
There is no FDA guidance on the use of Spironolactone and hydrochlorothiazide (patient information) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Spironolactone and hydrochlorothiazide (patient information) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Spironolactone and hydrochlorothiazide (patient information) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Spironolactone and hydrochlorothiazide (patient information) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Spironolactone and hydrochlorothiazide (patient information) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Spironolactone and hydrochlorothiazide (patient information) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Administration in the drug label.
Monitoring
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Spironolactone and hydrochlorothiazide (patient information) and IV administrations.
Overdosage
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Pharmacology in the drug label.
Mechanism of Action
- ALDACTAZIDE is a combination of two diuretic agents with different but complementary mechanisms and sites of action, thereby providing additive diuretic and antihypertensive effects. Additionally, the spironolactone component helps to minimize the potassium loss characteristically induced by the thiazide component.
- The diuretic effect of spironolactone is mediated through its action as a specific pharmacologic antagonist of aldosterone, primarily by competitive binding of receptors at the aldosterone-dependent sodium-potassium exchange site in the distal convoluted renal tubule. Hydrochlorothiazide promotes the excretion of sodium and water primarily by inhibiting their reabsorption in the cortical diluting segment of the distal renal tubule.
- ALDACTAZIDE is effective in significantly lowering the systolic and diastolic blood pressure in many patients with essential hypertension, even when aldosterone secretion is within normal limits.
- Both spironolactone and hydrochlorothiazide reduce exchangeable sodium, plasma volume, body weight, and blood pressure. The diuretic and antihypertensive effects of the individual components are potentiated when spironolactone and hydrochlorothiazide are given concurrently.
Structure
- ALDACTAZIDE oral tablets contain:
spironolactone . . . . . . . . . . . . . . . . . . . . 25 mg hydrochlorothiazide . . . . . . . . . . . . . . . . 25 mg
or
spironolactone . . . . . . . . . . . . . . . . . . . . 50 mg hydrochlorothiazide . . . . . . . . . . . . . . . . 50 mg
- Spironolactone (ALDACTONE®), an aldosterone antagonist, is 17-hydroxy-7α-mercapto-3-oxo-17α-pregn-4-ene-21-carboxylic acid γ-lactone acetate and has the following structural formula:
- Spironolactone is practically insoluble in water, soluble in alcohol, and freely soluble in benzene and in chloroform.
- Hydrochlorothiazide, a diuretic and antihypertensive, is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide and has the following structural formula:
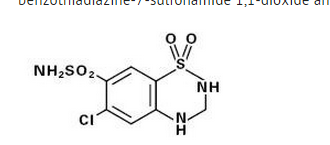
- Hydrochlorothiazide is slightly soluble in water and freely soluble in sodium hydroxide solution.
- Inactive ingredients include calcium sulfate, corn starch, flavor, hydroxypropyl cellulose, hypromellose, iron oxide, magnesium stearate, polyethylene glycol, povidone, and titanium dioxide.
Pharmacodynamics
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Pharmacodynamics in the drug label.
Pharmacokinetics
- Spironolactone is rapidly and extensively metabolized. Sulfur-containing products are the predominant metabolites and are thought to be primarily responsible, together with spironolactone, for the therapeutic effects of the drug. The following pharmacokinetic data were obtained from 12 healthy volunteers following the administration of 100 mg of spironolactone (ALDACTONE film-coated tablets) daily for 15 days. On the 15th day, spironolactone was given immediately after a low fat breakfast and blood was drawn thereafter.
- The pharmacological activity of spironolactone metabolites in man is not known. However, in the adrenalectomized rat the antimineralocorticoid activities of the metabolites C, TMS, and HTMS, relative to spironolactone, were 1.10, 1.28, and 0.32, respectively. Relative to spironolactone, their binding affinities to the aldosterone receptors in rat kidney slices were 0.19, 0.86, and 0.06, respectively.
- In humans, the potencies of TMS and 7-α-thiospirolactone in reversing the effects of the synthetic mineralocorticoid, fludrocortisone, on urinary electrolyte composition were 0.33 and 0.26, respectively, relative to spironolactone. However, since the serum concentrations of these steroids were not determined, their incomplete absorption and/or first-pass metabolism could not be ruled out as a reason for their reduced in vivo activities.
- Spironolactone and its metabolites are more than 90% bound to plasma proteins. The metabolites are excreted primarily in the urine and secondarily in bile.
- The effect of food on spironolactone absorption (two 100 mg ALDACTONE tablets) was assessed in a single dose study of 9 healthy, drug-free volunteers. Food increased the bioavailability of unmetabolized spironolactone by almost 100%. The clinical importance of this finding is not known.
- Hydrochlorothiazide is rapidly absorbed following oral administration. Onset of action of hydrochlorothiazide is observed within one hour and persists for 6 to 12 hours. Hydrochlorothiazide plasma concentrations attain peak levels at one to two hours and decline with a half-life of four to five hours. Hydrochlorothiazide undergoes only slight metabolic alteration and is excreted in urine. It is distributed throughout the extracellular space, with essentially no tissue accumulation except in the kidney.
Nonclinical Toxicology
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) How Supplied in the drug label.
Storage
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Spironolactone and hydrochlorothiazide (patient information) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Spironolactone and hydrochlorothiazide (patient information) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Spironolactone and hydrochlorothiazide (patient information) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Spironolactone and hydrochlorothiazide (patient information) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
IMPORTANT WARNING:
Spironolactone has caused tumors in laboratory animals. Talk to your doctor about the risks and benefits of using this medicine for your condition.
Why is this medication prescribed
The combination of spironolactone and hydrochlorothiazide, a 'water pill,' is used to treat high blood pressure and fluid retention caused by various conditions, including heart disease. It causes the kidneys to eliminate unneeded water and salt from the body into the urine.
This medicine is sometimes prescribed for other uses; ask your doctor or pharmacist for more information.
How should this medicine be used
The combination of spironolactone and hydrochlorothiazide comes as a tablet to take by mouth. It usually is taken once a day in the morning with food. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take spironolactone and hydrochlorothiazide exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
This medication controls high blood pressure but does not cure it. Continue to take spironolactone and hydrochlorothiazide even if you feel well. Do not stop taking spironolactone and hydrochlorothiazide without talking to your doctor.
What special precautions should I follow
Before taking spironolactone and hydrochlorothiazide:
- tell your doctor and pharmacist if you are allergic to spironolactone, hydrochlorothiazide, sulfa drugs, or any other drugs.
- tell your doctor and pharmacist what prescription and nonprescription medications you are taking, especially aspirin; captopril (Capoten); digoxin (Lanoxin); enalapril (Vasotec); lisinopril (Prinivil, Zestril); lithium (Eskalith, Lithobid); medications for arthritis, diabetes, or high blood pressure; potassium supplements; and vitamins. Do not take this medicine if you are taking amiloride or triamterene.
- tell your doctor if you have or have ever had diabetes, gout, or kidney or liver disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking spironolactone and hydrochlorothiazide, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking spironolactone and hydrochlorothiazide.
- you should know that this drug may make you drowsy. Do not drive a car or operate machinery until you know how this drug affects you.
- remember that alcohol can add to the drowsiness caused by this drug.
What special dietary instructions should I follow
Follow your doctor's directions for a low-salt or low-sodium diet and daily exercise program. Avoid potassium-containing salt substitutes. Limit your intake of potassium-rich foods (e.g., bananas, prunes, raisins, and orange juice). Ask your doctor for advice on how much of these foods you may have.
What should I do if I forget a dose? Return to top Take the missed dose as soon as you remember it. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Side effects
Mild side effects
Spironolactone and hydrochlorothiazide may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- upset stomach
- vomiting
- diarrhea
- loss of appetite
- stomach pain
- gas
- frequent urination
- dizziness
- headache
- enlarged or painful breasts
- irregular menstrual periods
- drowsiness
Severe side effects
If you experience any of the following symptoms, call your doctor immediately:
- muscle weakness or cramps
- rapid, excessive weight loss
- fatigue
- slow or irregular heartbeat
- sore throat
- unusual bruising or bleeding
- yellowing of the skin or eyes
- skin rash
- vomiting blood
- fever
- confusion
If you experience a serious side effect, you or your doctor may send a report to the Food and Drug Administration's (FDA) MedWatch Adverse Event Reporting program online [at http://www.fda.gov/MedWatch/report.htm] or by phone [1-800-332-1088].
What storage conditions are needed for this medicine
Keep this medicine in the container it came in, tightly closed, and out of reach of children. Store it at room temperature and away from excess heat and moisture (not in the bathroom). Throw away any medicine that is outdated or no longer needed. Talk to your pharmacist about the proper disposal of your medicine.
In case of emergency/overdose
In case of overdose, call your local poison control center at 1-800-222-1222. If the victim has collapsed or is not breathing, call local emergency services at 911.
What other information should I know
Keep all appointments with your doctor and the laboratory. Your blood pressure should be checked regularly, and blood tests should be done occasionally.
Do not let anyone else take your medicine. Ask your pharmacist any questions you have about refilling your prescription.
Brand names
- Aldactazide®
- Spironazide®
- Spirozide®