Sevoflurane
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sevoflurane is a general anesthetic that is FDA approved for the {{{indicationType}}} of general anesthesia. Common adverse reactions include cardiovascular: bradyarrhythmia (3% to 5% ), hypotension (4% to 11% ), gastrointestinal: nausea (25% ), vomiting (18% ), neurologic: somnolence (9% ), psychiatric: agitation (6% to 15%), respiratory: cough (5% to 11% ), interrupted breathing (2% to 6% ), other: shivering (6% ).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- General anesthesia: 0.5% to 3% concentration with or without concomitant use of nitrous oxide
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Sevoflurane in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Sevoflurane in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- General anesthesia: 0.5% to 3% concentration with or without concomitant use of nitrous oxide
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Sevoflurane in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Sevoflurane in pediatric patients.
Contraindications
- Sevoflurane can cause malignant hyperthermia. It should not be used in patients with known sensitivity to sevoflurane or to other halogenated agents nor in patients with known or suspected susceptibility to malignant hyperthermia.
Warnings
- Although data from controlled clinical studies at low flow rates are limited, findings taken from patient and animal studies suggest that there is a potential for renal injury which is presumed due to Compound A. Animal and human studies demonstrate that sevoflurane administered for more than 2 MAC·hours and at fresh gas flow rates of < 2 L/min may be associated with proteinuria and glycosuria.
- While a level of Compound A exposure at which clinical nephrotoxicity might be expected to occur has not been established, it is prudent to consider all of the factors leading to Compound A exposure in humans, especially duration of exposure, fresh gas flow rate, and concentration of sevoflurane. During sevoflurane anesthesia the clinician should adjust inspired concentration and fresh gas flow rate to minimize exposure to Compound A. To minimize exposure to Compound A, sevoflurane exposure should not exceed 2 MAC·hours at flow rates of 1 to < 2 L/min. Fresh gas flow rates < 1 L/min are not recommended.
- Because clinical experience in administering sevoflurane to patients with renal insufficiency (creatinine > 1.5 mg/dL) is limited, its safety in these patients has not been established.
- Sevoflurane may be associated with glycosuria and proteinuria when used for long procedures at low flow rates. The safety of low flow sevoflurane on renal function was evaluated in patients with normal preoperative renal function. One study compared sevoflurane (N = 98) to an active control (N = 90) administered for ≥ 2 hours at a fresh gas flow rate of ≤ 1 Liter/minute. Per study defined criteria (Hou et al.) one patient in the sevoflurane group developed elevations of creatinine, in addition to glycosuria and proteinuria. This patient received sevoflurane at fresh gas flow rates of ≤ 800 mL/minute. Using these same criteria, there were no patients in the active control group who developed treatment emergent elevations in serum creatinine.
- Sevoflurane may present an increased risk in patients with known sensitivity to volatile halogenated anesthetic agents. KOH containing CO2 absorbents are not recommended for use with sevoflurane.
- Reports of QT prolongation, associated with torsade de pointes (in exceptional cases, fatal), have been received. Caution should be exercised when administering sevoflurane to susceptible patients (e.g. patients with congenital Long QT Syndrome or patients taking drugs that can prolong the QT interval).
Malignant Hyperthermia
- In susceptible individuals, potent inhalation anesthetic agents, including sevoflurane, may trigger a skeletal muscle hypermetabolic state leading to high oxygen demand and the clinical syndrome known as malignant hyperthermia. Sevoflurane can induce malignant hyperthermia in genetically susceptible individuals, such as those with certain inherited ryanodine receptor mutations. The clinical syndrome is signaled by hypercapnia, and may include muscle rigidity, tachycardia, tachypnea, cyanosis, arrhythmias, and/or unstable blood pressure. Some of these nonspecific signs may also appear during light anesthesia, acute hypoxia, hypercapnia, and hypovolemia.
- In clinical trials, one case of malignant hyperthermia was reported. In addition, there have been postmarketing reports of malignant hyperthermia. Some of these cases have been fatal.
- Treatment of malignant hyperthermia includes discontinuation of triggering agents (e.g., sevoflurane), administration of intravenous dantrolene sodium (consult prescribing information for intravenous dantrolene sodium for additional information on patient management), and application of supportive therapy. Supportive therapy may include efforts to restore body temperature, respiratory and circulatory support as indicated, and management of electrolyte-fluid-acid-base abnormalities. Renal failure may appear later, and urine flow should be monitored and sustained if possible.
Perioperative Hyperkalemia
- Use of inhaled anesthetic agents has been associated with rare increases in serum potassium levels that have resulted in cardiac arrhythmias and death in pediatric patients during the postoperative period. Patients with latent as well as overt neuromuscular disease, particularly Duchenne muscular dystrophy, appear to be most vulnerable. Concomitant use of succinylcholine has been associated with most, but not all, of these cases. These patients also experienced significant elevations in serum creatine kinase levels and, in some cases, changes in urine consistent with myoglobinuria. Despite the similarity in presentation to malignant hyperthermia, none of these patients exhibited signs or symptoms of muscle rigidity or hypermetabolic state. Early and aggressive intervention to treat the hyperkalemia and resistant arrhythmias is recommended; as is subsequent evaluation for latent neuromuscular disease.
Adverse Reactions
Clinical Trials Experience
- Adverse events are derived from controlled clinical trials conducted in the United States, Canada, and Europe. The reference drugs were isoflurane, enflurane, and propofol in adults and halothane in pediatric patients. The studies were conducted using a variety of premedications, other anesthetics, and surgical procedures of varying length. Most adverse events reported were mild and transient, and may reflect the surgical procedures, patient characteristics (including disease) and/or medications administered.
- Of the 5182 patients enrolled in the clinical trials, 2906 were exposed to sevoflurane, including 118 adults and 507 pediatric patients who underwent mask induction. Each patient was counted once for each type of adverse event. Adverse events reported in patients in clinical trials and considered to be possibly or probably related to sevoflurane are presented within each body system in order of decreasing frequency in the following listings. One case of malignant hyperthermia was reported in pre-registration clinical trials.
- Adverse Events During the Induction Period (from Onset of Anesthesia by Mask Induction to Surgical Incision) Incidence > 1%
- Adult Patients (N = 118)
Cardiovascular
- Bradycardia 5%, hypotension 4%, tachycardia 2%
Nervous System
- Agitation 7%
Respiratory System
- Laryngospasm 8%, airway obstruction 8%, breathholding 5%, cough increased 5%
Pediatric Patients (N = 507)
Cardiovascular
- Tachycardia 6%, hypotension 4%
Nervous System
- Agitation 15%
Respiratory System
- Breathholding 5%, cough increased 5%, laryngospasm 3%, apnea 2%
Digestive System
- Increased salivation 2%
Adverse Events During Maintenance and Emergence Periods, Incidence > 1% (N = 2906)
Body as a whole
- Fever 1%, shivering 6%, hypothermia 1%, movement 1%, headache 1%
Cardiovascular
- Hypotension 11%, hypertension 2%, bradycardia 5%, tachycardia 2%
Nervous System
- Somnolence 9%, agitation 9%, dizziness 4%, increased salivation 4%
Digestive System
Respiratory System
- Cough increased 11%, Breathholding 2%, Laryngospasm 2%
Adverse Events, All Patients in Clinical Trials (N = 2906), All Anesthetic Periods, Incidence < 1% (Reported in 3 or More Patients)
Body as a whole
Cardiovascular
- Arrhythmia, ventricular extrasystoles, supraventricular extrasystoles, complete av block, bigeminy, hemorrhage, inverted t wave, atrial fibrillation, atrial arrhythmia, second degree av block, syncope, s-t depressed
Nervous system
Respiratory System
- Sputum Increased, apnea, hypoxia, wheezing, bronchospasm, hyperventilation, pharyngitis, hiccup, hypoventilation, dyspnea, stridor
Metabolism and Nutrition
- Increases in LDH, AST, ALT, BUN, alkaline phosphatase, creatinine, bilirubinemia, glycosuria, fluorosis, albuminuria, hypophosphatemia, acidosis, hyperglycemia
Hemic and Lymphatic System
Skin and Special Senses
Urogenital
- Urination impaired, urine abnormality, urinary retention, oliguria
- See Warnings for information regarding malignant hyperthermia.
Postmarketing Experience
- The following adverse events have been identified during post-approval use of Ultane (sevoflurane USP). Due to the spontaneous nature of these reports, the actual incidence and relationship of Ultane to these events cannot be established with certainty.
CNS
- Seizures — Post-marketing reports indicate that sevoflurane use has been associated with seizures. The majority of cases were in children and young adults, most of whom had no medical history of seizures. Several cases reported no concomitant medications, and at least one case was confirmed by EEG. Although many cases were single seizures that resolved spontaneously or after treatment, cases of multiple seizures have also been reported. Seizures have occurred during, or soon after sevoflurane induction, during emergence, and during post-operative recovery up to a day following anesthesia.
Cardiac
Hepatic
- Cases of mild, moderate and severe post-operative hepatic dysfunction or hepatitis with or without jaundice have been reported. Histological evidence was not provided for any of the reported hepatitis cases. In most of these cases, patients had underlying hepatic conditions or were under treatment with drugs known to cause hepatic dysfunction. Most of the reported events were transient and resolved spontaneously (see Precautions).
- Hepatic necrosis
- Hepatic failure
Other
- Malignant hyperthermia (see Contraindications and Warnings)
- Allergic reactions, such as rash, urticaria, pruritus, bronchospasm, anaphylactic or anaphylactoid reactions (see Contraindications)
- Reports of hypersensitivity (including contact dermatitis, rash, dyspnea, wheezing, chest discomfort, swelling face, or anaphylactic reaction) have been received, particularly in association with long-term occupational exposure to inhaled anesthetic agents, including sevoflurane (see Occupational caution).
Laboratory Findings
- Transient elevations in glucose, liver function tests, and white blood cell count may occur as with use of other anesthetic agents.
Drug Interactions
- In clinical trials, no significant adverse reactions occurred with other drugs commonly used in the perioperative period, including: central nervous system depressants, autonomic drugs, skeletal muscle relaxants, anti-infective agents, hormones and synthetic substitutes, blood derivatives, and cardiovascular drugs.
Intravenous Anesthetics
- Sevoflurane administration is compatible with barbiturates, propofol, and other commonly used intravenous anesthetics.
Benzodiazepines and Opioids
- Benzodiazepines and opioids would be expected to decrease the MAC of sevoflurane in the same manner as with other inhalational anesthetics. Sevoflurane administration is compatible with benzodiazepines and opioids as commonly used in surgical practice.
Nitrous Oxide
- As with other halogenated volatile anesthetics, the anesthetic requirement for sevoflurane is decreased when administered in combination with nitrous oxide. Using 50% N2O, the MAC equivalent dose requirement is reduced approximately 50% in adults, and approximately 25% in pediatric patients (see Dosage and administration).
Neuromuscular Blocking Agents
- As is the case with other volatile anesthetics, sevoflurane increases both the intensity and duration of neuromuscular blockade induced by nondepolarizing muscle relaxants. When used to supplement alfentanil-N2O anesthesia, sevoflurane and isoflurane equally potentiate neuromuscular block induced with pancuronium, vecuronium or atracurium. Therefore, during sevoflurane anesthesia, the dosage adjustments for these muscle relaxants are similar to those required with isoflurane.
- Potentiation of neuromuscular blocking agents requires equilibration of muscle with delivered partial pressure of sevoflurane. Reduced doses of neuromuscular blocking agents during induction of anesthesia may result in delayed onset of conditions suitable for endotracheal intubation or inadequate muscle relaxation.
- Among available nondepolarizing agents, only vecuronium, pancuronium and atracurium interactions have been studied during sevoflurane anesthesia. In the absence of specific guidelines:
- For endotracheal intubation, do not reduce the dose of nondepolarizing muscle relaxants.
- During maintenance of anesthesia, the required dose of nondepolarizing muscle relaxants is likely to be reduced compared to that during N2O/opioid anesthesia. Administration of supplemental doses of muscle relaxants should be guided by the response to nerve stimulation.
- The effect of sevoflurane on the duration of depolarizing neuromuscular blockade induced by succinylcholine has not been studied.
Hepatic Function
- Results of evaluations of laboratory parameters (e.g., ALT, AST, alkaline phosphatase, and total bilirubin, etc.), as well as investigator-reported incidence of adverse events relating to liver function, demonstrate that sevoflurane can be administered to patients with normal or mild-to-moderately impaired hepatic function. However, patients with severe hepatic dysfunction were not investigated.
- Occasional cases of transient changes in postoperative hepatic function tests were reported with both sevoflurane and reference agents. Sevoflurane was found to be comparable to isoflurane with regard to these changes in hepatic function.
- Very rare cases of mild, moderate and severe post-operative hepatic dysfunction or hepatitis with or without jaundice have been reported from postmarketing experiences. Clinical judgement should be exercised when sevoflurane is used in patients with underlying hepatic conditions or under treatment with drugs known to cause hepatic dysfunction (see ADVERSE REACTIONS).
- It has been reported that previous exposure to halogenated hydrocarbon anesthetics may increase the potential for hepatic injury.
Desiccated CO2 Absorbents
- An exothermic reaction occurs when sevoflurane is exposed to CO2 absorbents. This reaction is increased when the CO2 absorbent becomes desiccated, such as after an extended period of dry gas flow through the CO2 absorbent canisters. Rare cases of extreme heat, smoke, and/or spontaneous fire in the anesthesia breathing circuit have been reported during sevoflurane use in conjunction with the use of desiccated CO2 absorbent, specifically those containing potassium hydroxide (e.g. Baralyme). KOH containing CO2 absorbents are not recommended for use with sevoflurane. An unusually delayed rise or unexpected decline of inspired sevoflurane concentration compared to the vaporizer setting may be associated with excessive heating of the CO2 absorbent and chemical breakdown of sevoflurane.
- As with other inhalational anesthetics, degradation and production of degradation products can occur when sevoflurane is exposed to desiccated absorbents. When a clinician suspects that the CO2 absorbent may be desiccated, it should be replaced. The color indicator of most CO2 absorbents may not change upon desiccation. Therefore, the lack of significant color change should not be taken as an assurance of adequate hydration. CO2 absorbents should be replaced routinely regardless of the state of the color indicator.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in rats and rabbits at doses up to 1 MAC (minimum alveolar concentration) without CO2 absorbent and have revealed no evidence of impaired fertility or harm to the fetus due to sevoflurane at 0.3 MAC, the highest nontoxic dose. Developmental and reproductive toxicity studies of sevoflurane in animals in the presence of strong alkalies (i.e., degradation of sevoflurane and production of Compound A) have not been conducted. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, sevoflurane should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sevoflurane in women who are pregnant.
Labor and Delivery
- Sevoflurane has been used as part of general anesthesia for elective cesarean section in 29 women. There were no untoward effects in mother or neonate (see Pharmacodynamics - Clinical Trials). The safety of sevoflurane in labor and delivery has not been demonstrated.
Nursing Mothers
- The concentrations of sevoflurane in milk are probably of no clinical importance 24 hours after anesthesia. Because of rapid washout, sevoflurane concentrations in milk are predicted to be below those found with many other volatile anesthetics.
Pediatric Use
- Induction and maintenance of general anesthesia with sevoflurane have been established in controlled clinical trials in pediatric patients aged 1 to 18 years (see Pharmacodynamics - Clinical Trials and Adverse reactions). Sevoflurane has a nonpungent odor and is suitable for mask induction in pediatric patients.
- The concentration of sevoflurane required for maintenance of general anesthesia is age dependent. When used in combination with nitrous oxide, the MAC equivalent dose of sevoflurane should be reduced in pediatric patients. MAC in premature infants has not been determined (see Precautions - Drug Interactions and Dosage and administration for recommendations in pediatric patients 1 day of age and older).
- The use of sevoflurane has been associated with seizures (see PRECAUTIONS and ADVERSE REACTIONS). The majority of these have occurred in children and young adults starting from 2 months of age, most of whom had no predisposing risk factors. Clinical judgement should be exercised when using sevoflurane in patients who may be at risk for seizures.
Geriatic Use
- MAC decreases with increasing age. The average concentration of sevoflurane to achieve MAC in an 80 year old is approximately 50% of that required in a 20 year old.
Gender
There is no FDA guidance on the use of Sevoflurane with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sevoflurane with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sevoflurane in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sevoflurane in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sevoflurane in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sevoflurane in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Sevoflurane Administration in the drug label.
Monitoring
There is limited information regarding Sevoflurane Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sevoflurane and IV administrations.
Overdosage
- In the event of overdosage, or what may appear to be overdosage, the following action should be taken: discontinue administration of sevoflurane, maintain a patent airway, initiate assisted or controlled ventilation with oxygen, and maintain adequate cardiovascular function.
Pharmacology
| |
| |
Sevoflurane
| |
| Systematic (IUPAC) name | |
| 1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)propane | |
| Identifiers | |
| CAS number | |
| ATC code | N01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 200.055 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | inhaled |
Mechanism of Action
- Sevoflurane is an inhalational anesthetic agent for use in induction and maintenance of general anesthesia. Minimum alveolar concentration (MAC) of sevoflurane in oxygen for a 40-year-old adult is 2.1%. The MAC of sevoflurane decreases with age (see Dosage and administration for details).
Structure
- ULTANE (sevoflurane), volatile liquid for inhalation, a nonflammable and nonexplosive liquid administered by vaporization, is a halogenated general inhalation anesthetic drug. Sevoflurane is fluoromethyl 2,2,2,-trifluoro-1-(trifluoromethyl) ethyl ether and its structural formula is:
- Sevoflurane is nonflammable and nonexplosive as defined by the requirements of International Electrotechnical Commission 601-2-13.
- Sevoflurane is a clear, colorless, liquid containing no additives. Sevoflurane is not corrosive to stainless steel, brass, aluminum, nickel-plated brass, chrome-plated brass or copper beryllium. Sevoflurane is nonpungent. It is miscible with ethanol, ether, chloroform, and benzene, and it is slightly soluble in water. Sevoflurane is stable when stored under normal room lighting conditions according to instructions. No discernible degradation of sevoflurane occurs in the presence of strong acids or heat. When in contact with alkaline CO2 absorbents (e.g Baralyme® and to a lesser extent soda lime) within the anesthesia machine, sevoflurane can undergo degradation under certain conditions. Degradation of sevoflurane is minimal, and degradants are either undetectable or present in non-toxic amounts when used as directed with fresh absorbents. Sevoflurane degradation and subsequent degradant formation are enhanced by increasing absorbent temperature increased sevoflurane concentration, decreased fresh gas flow and desiccated CO2 absorbents (especially with potassium hydroxide containing absorbents e.g. Baralyme).
- Sevoflurane alkaline degradation occurs by two pathways. The first results from the loss of hydrogen fluoride with the formation of pentafluoroisopropenyl fluoromethyl ether, (PIFE, C4H2F6O), also known as Compound A, and trace amounts of pentafluoromethoxy isopropyl fluoromethyl ether, (PMFE, C5H6F6O), also known as Compound B. The second pathway for degradation of sevoflurane, which occurs primarily in the presence of desiccated CO2 absorbents, is discussed later.
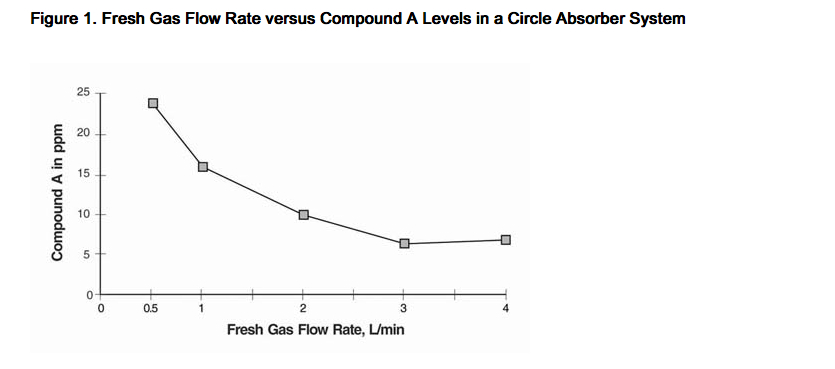
- In the first pathway, the defluorination pathway, the production of degradants in the anesthesia circuit results from the extraction of the acidic proton in the presence of a strong base (KOH and/or NaOH) forming an alkene (Compound A) from sevoflurane similar to formation of 2-bromo-2-chloro-1,1-difluoro ethylene (BCDFE) from halothane. Laboratory simulations have shown that the concentration of these degradants is inversely correlated with the fresh gas flow rate (See Figure 1).

- Since the reaction of carbon dioxide with absorbents is exothermic, the temperature increase will be determined by quantities of CO2 absorbed, which in turn will depend on fresh gas flow in the anesthesia circle system, metabolic status of the patient, and ventilation. The relationship of temperature produced by varying levels of CO2 and Compound A production is illustrated in the following in vitro simulation where CO2 was added to a circle absorber system.
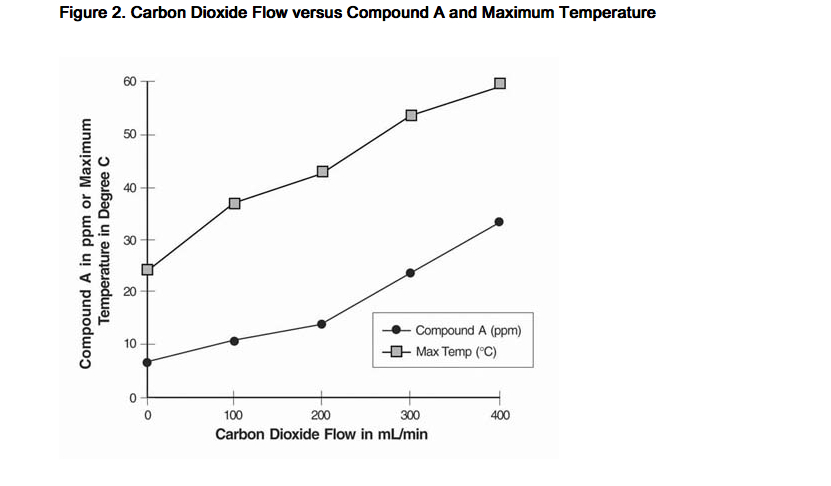
- Compound A concentration in a circle absorber system increases as a function of increasing CO2 absorbent temperature and composition (Baralyme producing higher levels than soda lime), increased body temperature, and increased minute ventilation, and decreasing fresh gas flow rates. It has been reported that the concentration of Compound A increases significantly with prolonged dehydration of Baralyme. Compound A exposure in patients also has been shown to rise with increased sevoflurane concentrations and duration of anesthesia. In a clinical study in which sevoflurane was administered to patients under low flow conditions for ≥ 2 hours at flow rates of 1 Liter/minute, Compound A levels were measured in an effort to determine the relationship between MAC hours and Compound A levels produced. The relationship between Compound A levels and sevoflurane exposure are shown in Figure 2a.
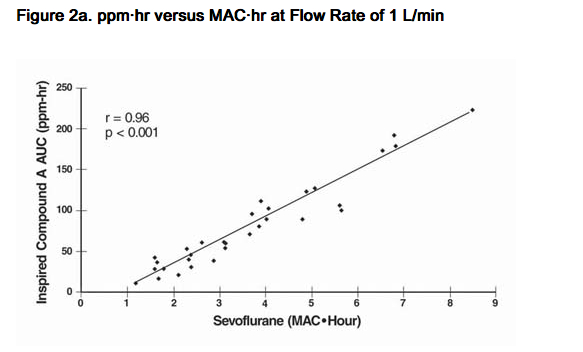
- Compound A has been shown to be nephrotoxic in rats after exposures that have varied in duration from one to three hours. No histopathologic change was seen at a concentration of up to 270 ppm for one hour. Sporadic single cell necrosis of proximal tubule cells has been reported at a concentration of 114 ppm after a 3-hour exposure to Compound A in rats. The LC50 reported at 1 hour is 1050-1090 ppm (male-female) and, at 3 hours, 350-490 ppm (male-female).
- An experiment was performed comparing sevoflurane plus 75 or 100 ppm Compound A with an active control to evaluate the potential nephrotoxicity of Compound A in non-human primates. A single 8-hour exposure of Sevoflurane in the presence of Compound A produced single-cell renal tubular degeneration and single-cell necrosis in cynomolgus monkeys. These changes are consistent with the increased urinary protein, glucose level and enzymic activity noted on days one and three on the clinical pathology evaluation. This nephrotoxicity produced by Compound A is dose and duration of exposure dependent.
- At a fresh gas flow rate of 1 L/min, mean maximum concentrations of Compound A in the anesthesia circuit in clinical settings are approximately 20 ppm (0.002%) with soda lime and 30 ppm (0.003%) with Baralyme in adult patients; mean maximum concentrations in pediatric patients with soda lime are about half those found in adults. The highest concentration observed in a single patient with Baralyme was 61 ppm (0.0061%) and 32 ppm (0.0032%) with soda lime. The levels of Compound A at which toxicity occurs in humans is not known.
- The second pathway for degradation of sevoflurane occurs primarily in the presence of desiccated CO2 absorbents and leads to the dissociation of sevoflurane into hexafluoroisopropanol (HFIP) and formaldehyde. HFIP is inactive, non-genotoxic, rapidly glucuronidated and cleared by the liver. Formaldehyde is present during normal metabolic processes. Upon exposure to a highly desiccated absorbent, formaldehyde can further degrade into methanol and formate. Formate can contribute to the formation of carbon monoxide in the presence of high temperature that can be associated with desiccated Baralyme®. Methanol can react with Compound A to form the methoxy addition product Compound B. Compound B can undergo further HF elimination to form Compounds C, D, and E.
- Sevoflurane degradants were observed in the respiratory circuit of an experimental anesthesia machine using desiccated CO2 absorbents and maximum sevoflurane concentrations (8%) for extended periods of time (> 2 hours). Concentrations of formaldehyde observed with desiccated soda lime in this experimental anesthesia respiratory circuit were consistent with levels that could potentially result in respiratory irritation. Although KOH containing CO2 absorbents are no longer commercially available, in the laboratory experiments, exposure of sevoflurane to the desiccated KOH containing CO2 absorbent, Baralyme, resulted in the detection of substantially greater degradant levels.
Pharmacodynamics
- Changes in the depth of sevoflurane anesthesia rapidly follow changes in the inspired concentration.
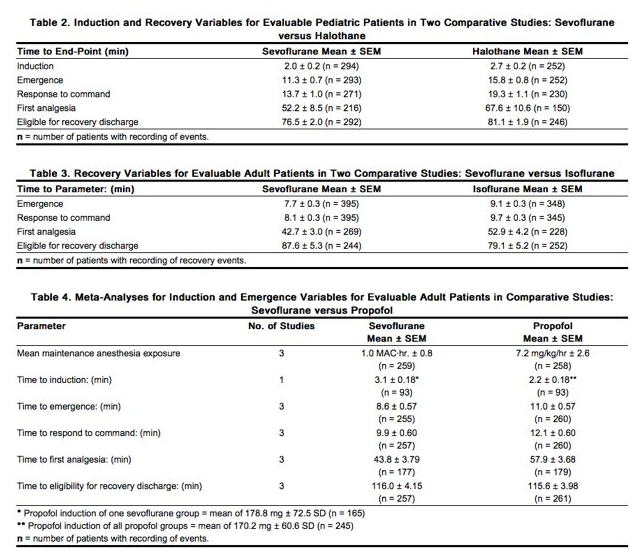
- In the sevoflurane clinical program, the following recovery variables were evaluated:
- Time to events measured from the end of study drug:
- Time to removal of the endotracheal tube (extubation time)
- Time required for the patient to open his/her eyes on verbal command (emergence time)
- Time to respond to simple command (e.g., squeeze my hand) or demonstrates purposeful movement (response to command time, orientation time)
- Recovery of cognitive function and motor coordination was evaluated based on:
- Psychomotor performance tests (Digit Symbol Substitution Test [DSST], Treiger Dot Test)
- The results of subjective (Visual Analog Scale [VAS]) and objective (objective pain-discomfort scale [OPDS]) measurements
- Time to administration of the first post-anesthesia analgesic medication
- Assessments of post-anesthesia patient status
- Other recovery times were:
- Time to achieve an Aldrete Score of ≥ 8
- Time required for the patient to be eligible for discharge from the recovery area, per standard criteria at site
- Time when the patient was eligible for discharge from the hospital
- Time when the patient was able to sit up or stand without dizziness
- Some of these variables are summarized as follows:
Cardiovascular Effects
- Sevoflurane was studied in 14 healthy volunteers (18-35 years old) comparing sevoflurane-O2 (Sevo/O2) to sevoflurane-N2O/O2 (Sevo/N2O/O2) during 7 hours of anesthesia. During controlled ventilation, hemodynamic parameters measured are shown in Figures 7-10:
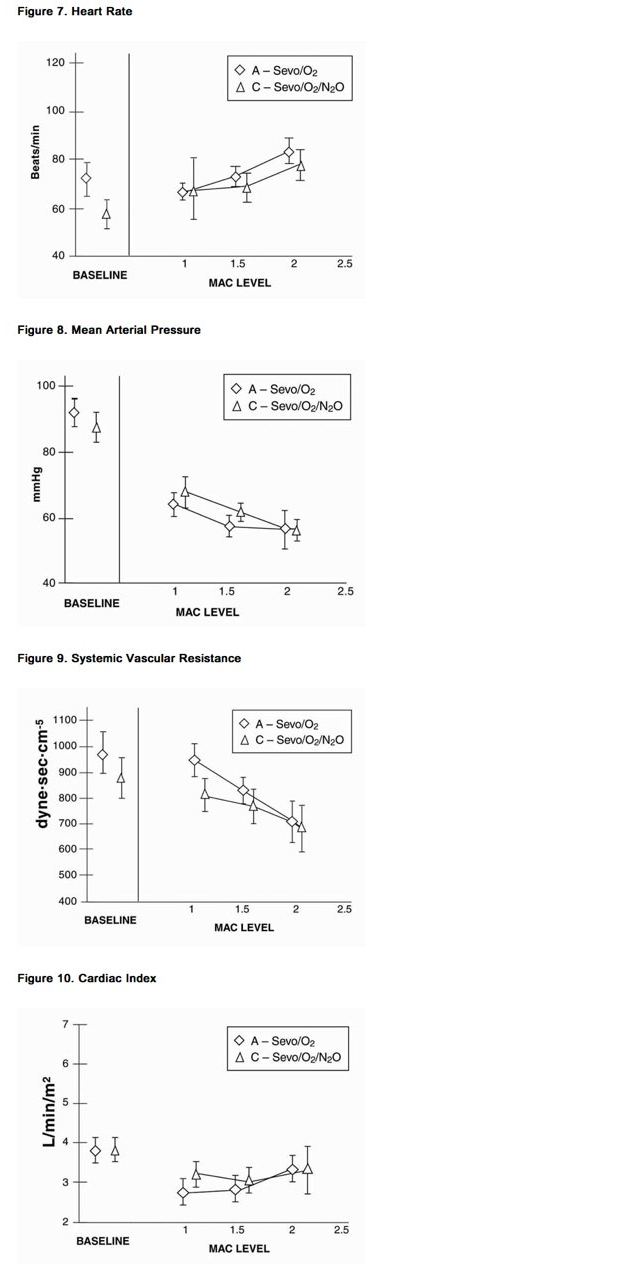
- Sevoflurane is a dose-related cardiac depressant. Sevoflurane does not produce increases in heart rate at doses less than 2 MAC.
- A study investigating the epinephrine induced arrhythmogenic effect of sevoflurane versus isoflurane in adult patients undergoing transsphenoidal hypophysectomy demonstrated that the threshold dose of epinephrine (i.e., the dose at which the first sign of arrhythmia was observed) producing multiple ventricular arrhythmias was 5 mcg/kg with both sevoflurane and isoflurane. Consequently, the interaction of sevoflurane with epinephrine appears to be equal to that seen with isoflurane.
Pharmacokinetics
Uptake and Distribution
Solubility
- Because of the low solubility of sevoflurane in blood (blood/gas partition coefficient @ 37°C = 0.63-0.69), a minimal amount of sevoflurane is required to be dissolved in the blood before the alveolar partial pressure is in equilibrium with the arterial partial pressure. Therefore there is a rapid rate of increase in the alveolar (end-tidal) concentration (FA) toward the inspired concentration (FI) during induction.
Induction of Anesthesia
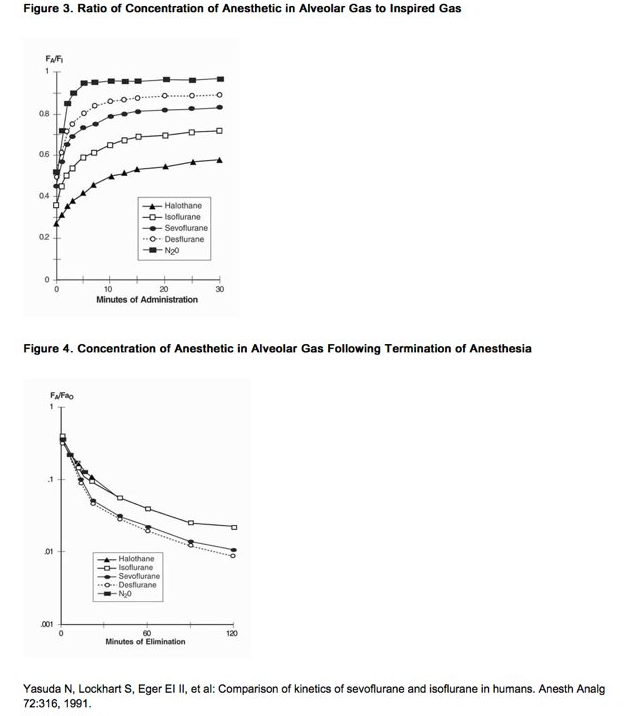
- In a study in which seven healthy male volunteers were administered 70% N2O/30%O2 for 30 minutes followed by 1.0% sevoflurane and 0.6% isoflurane for another 30 minutes the FA/FI ratio was greater for sevoflurane than isoflurane at all time points. The time for the concentration in the alveoli to reach 50% of the inspired concentration was 4-8 minutes for isoflurane and approximately 1 minute for sevoflurane.
- FA/FI data from this study were compared with FA/FI data of other halogenated anesthetic agents from another study. When all data were normalized to isoflurane, the uptake and distribution of sevoflurane was shown to be faster than isoflurane and halothane, but slower than desflurane. The results are depicted in Figure 3.
Recovery from Anesthesia
- The low solubility of sevoflurane facilitates rapid elimination via the lungs. The rate of elimination is quantified as the rate of change of the alveolar (end-tidal) concentration following termination of anesthesia (FA), relative to the last alveolar concentration (FaO) measured immediately before discontinuance of the anesthetic. In the healthy volunteer study described above, rate of elimination of sevoflurane was similar compared with desflurane, but faster compared with either halothane or isoflurane. These results are depicted in Figure 4.
- Figure 3. Ratio of Concentration of Anesthetic in Alveolar Gas to Inspired Gas
Protein Binding
- The effects of sevoflurane on the displacement of drugs from serum and tissue proteins have not been investigated. Other fluorinated volatile anesthetics have been shown to displace drugs from serum and tissue proteins in vitro. The clinical significance of this is unknown. Clinical studies have shown no untoward effects when sevoflurane is administered to patients taking drugs that are highly bound and have a small volume of distribution (e.g., phenytoin).
Metabolism
- Sevoflurane is metabolized by cytochrome P450 2E1, to hexafluoroisopropanol (HFIP) with release of inorganic fluoride and CO2. Once formed HFIP is rapidly conjugated with glucuronic acid and eliminated as a urinary metabolite. No other metabolic pathways for sevoflurane have been identified. In vivo metabolism studies suggest that approximately 5% of the sevoflurane dose may be metabolized.
- Cytochrome P450 2E1 is the principal isoform identified for sevoflurane metabolism and this may be induced by chronic exposure to isoniazid and ethanol. This is similar to the metabolism of isoflurane and enflurane and is distinct from that of methoxyflurane which is metabolized via a variety of cytochrome P450 isoforms. The metabolism of sevoflurane is not inducible by barbiturates. As shown in Figure 5, inorganic fluoride concentrations peak within 2 hours of the end of sevoflurane anesthesia and return to baseline concentrations within 48 hours post-anesthesia in the majority of cases (67%). The rapid and extensive pulmonary elimination of sevoflurane minimizes the amount of anesthetic available for metabolism.
- Figure 5. Serum Inorganic Fluoride Concentrations for Sevoflurane and Other Volatile Anesthetics
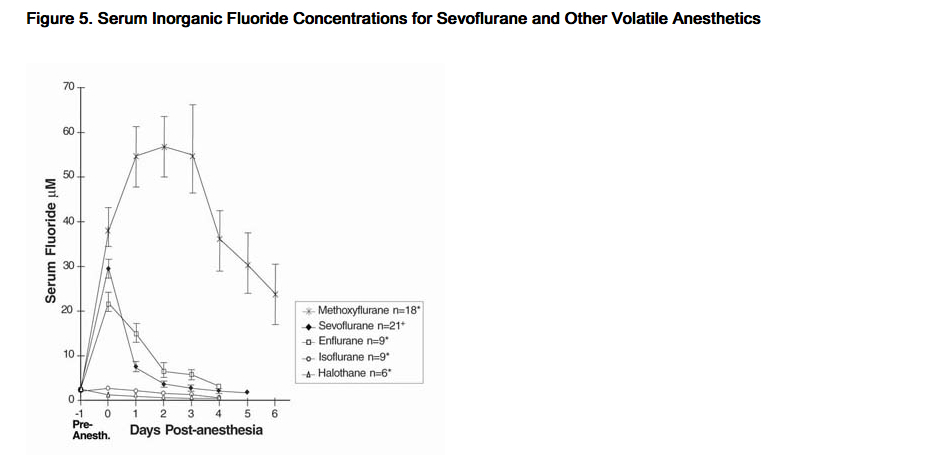
- Cousins M.J., Greenstein L.R., Hitt B.A., et al: Metabolism and renal effects of enflurane in man. Anesthesiology 44:44; 1976* and Sevo-93-044+.
- Legend:
- Pre-Anesth. = Pre-anesthesia
Elimination=
- Up to 3.5% of the sevoflurane dose appears in the urine as inorganic fluoride. Studies on fluoride indicate that up to 50% of fluoride clearance is nonrenal (via fluoride being taken up into bone).
Pharmacokinetics of Fluoride Ion
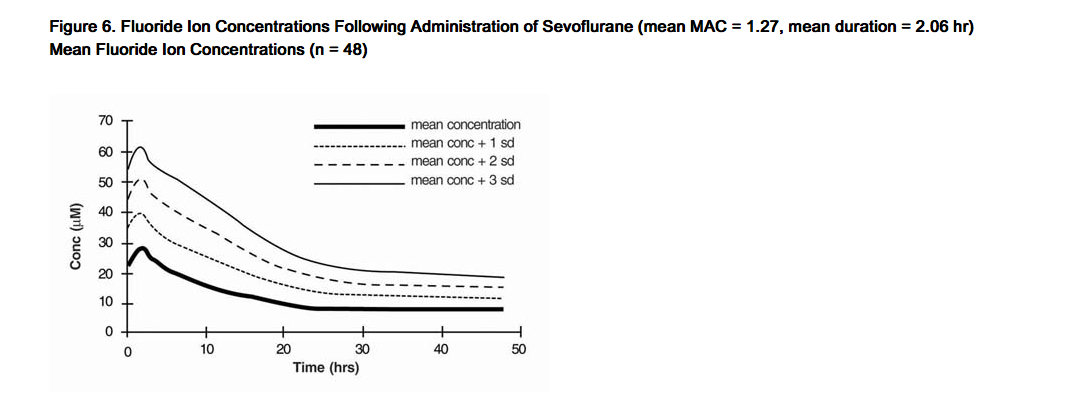
- Fluoride ion concentrations are influenced by the duration of anesthesia, the concentration of sevoflurane administered, and the composition of the anesthetic gas mixture. In studies where anesthesia was maintained purely with sevoflurane for periods ranging from 1 to 6 hours, peak fluoride concentrations ranged between 12 µM and 90 µM. As shown in Figure 6, peak concentrations occur within 2 hours of the end of anesthesia and are less than 25 µM (475 ng/mL) for the majority of the population after 10 hours. The half-life is in the range of 15-23 hours.
- It has been reported that following administration of methoxyflurane, serum inorganic fluoride concentrations > 50 µM were correlated with the development of vasopressin-resistant, polyuric, renal failure. In clinical trials with sevoflurane, there were no reports of toxicity associated with elevated fluoride ion levels.
- Figure 6. Fluoride Ion Concentrations Following Administration of Sevoflurane (mean MAC = 1.27, mean duration = 2.06 hr) Mean Fluoride Ion Concentrations (n = 48)
Fluoride Concentrations After Repeat Exposure and in Special Populations
- Fluoride concentrations have been measured after single, extended, and repeat exposure to sevoflurane in normal surgical and special patient populations, and pharmacokinetic parameters were determined.
- Compared with healthy individuals, the fluoride ion half-life was prolonged in patients with renal impairment, but not in the elderly. A study in 8 patients with hepatic impairment suggests a slight prolongation of the half-life. The mean half-life in patients with renal impairment averaged approximately 33 hours (range 21-61 hours) as compared to a mean of approximately 21 hours (range 10-48 hours) in normal healthy individuals. The mean half-life in the elderly (greater than 65 years) approximated 24 hours (range 18-72 hours). The mean half-life in individuals with hepatic impairment was 23 hours (range 16-47 hours). Mean maximal fluoride values (Cmax) determined in individual studies of special populations are displayed below.
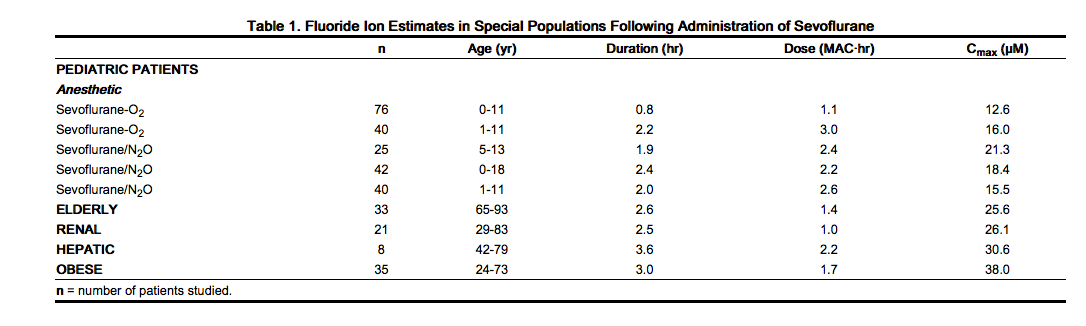
Nonclinical Toxicology
There is limited information regarding Sevoflurane Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sevoflurane Clinical Studies in the drug label.
How Supplied
- ULTANE (sevoflurane), Volatile Liquid for Inhalation, is packaged in amber colored bottles containing 250 mL sevoflurane, List 4456, NDC # 0074-4456-04 (plastic).
Storage
- Store at controlled room temperature, 15° - 30°C (59° - 86°F). See USP.
- Product of Japan
- Product inquiries should be directed to AbbVie Inc., North Chicago, IL 60064, USA
- Manufactured by:
- AbbVie Inc., North Chicago, IL 60064, USA under license from Maruishi Pharmaceutical Company LTD. 2-3-5, Fushimi-machi, Chuo-Ku, Osaka , Japan.
- ©AbbVie Inc. 2014
- 03-A903 March 2014
- NDC 0074–4456–04
- 250 mL
- ULTANE® sevoflurane
- Inhalation anesthetic
- Product of Japan.
- Rx only
abbvie
Images
Drug Images
{{#ask: Page Name::Sevoflurane |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sevoflurane |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sevoflurane Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sevoflurane interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sevoflurane Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sevoflurane Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Sevoflurane |Label Name=Sevoflurane label.png
}}