Sandbox:Roukoz
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Roukoz A. Karam, M.D.[2]
Overview
Protein S deficiency is an autosomal dominant thrombophilia, which leads to an increased risk of thromboembolic events. Protein S is a vitamin K-dependent glycoprotein and plays a role in anticoagulation. It is mainly a cofactor to the activated protein C (APC), which inactivates coagulation factors Va and VIIa and thereby controlling the coagulation cascade.
Historical Perspective
- Protein S was first discovered and purified in Seattle, Washington in 1979, and it was arbitrarily named protein S after the city it was discovered in.
- The function of this protein was still unknown; however, it was hypothesized that protein S plays a role in activating protein C.
- Protein S deficiency was first discovered in 1984 when two related individuals with recurrent thromboembolic events and normal coagulation tests were studied. At the time, protein C deficiency was usually associated with recurrent familial thrombosis. These individuals were found to have diminished anticoagulation activity with normal coagulation tests (including a normal protein C level), and when purified human protein S was added to their plasma, effective anticoagulation was restored. [1]
Classification
Protein S deficiency can be subdivided into three types depending on whether the abnormality affects total protein S level, free protein S level, and/or protein S function:[2]
- Type I: Reduced total protein S, free protein S, and protein S function
It is the classic form of hereditary protein S deficiency. Total protein S levels drop to approximately 50% of normal values while free protein S levels collapse to almost 15% of the normal. On a genetic level, type I deficiency usually results from missense or nonsense mutations. On few occasions, microinsertions, microdeletions, and splice site mutations have occurred with this type. [3]
- Type II: Normal total and free protein S, reduced protein S function
This form results from a qualitative defect and is very rare. The genetics behind this type isn't certain; however, some reports have linked it to missense mutations affecting the protein S's ability to bind to the activated protein C. [4] [5]
- Type III: Normal total protein S, reduced free protein S and protein S function
This is a quantitative defect.
| Type | Total Protein S | Free Protein S | Protein S Function |
|---|---|---|---|
| I | ↓ | ↓ | ↓ |
| II | ↔ | ↔ | ↓ |
| III | ↔ | ↓ | ↓ |
Pathophysiology
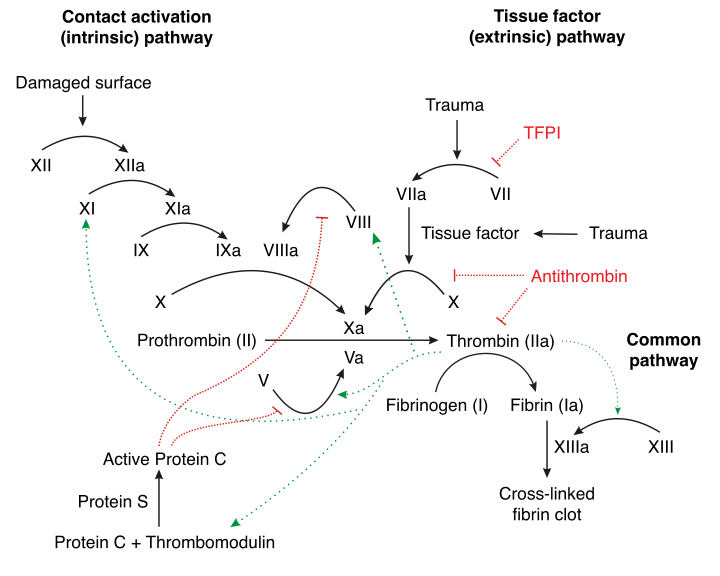 |
- Protein S is a natural anticoagulant that works with other proteins to regulate coagulation in the body.
- After it gets produced by the hepatocytes, endothelial cells, and megakaryocytes, protein S undergoes activation via vitamin K-dependent gamma-carboxylation. [7]
- The vitamin K-dependent gamma-carboxyalse enzyme acts by modifying the glutamic acid residues in protein S to gamma-carboxyglutamic acid residues.
- These gamma-carboxyglutamic acid residues are needed to ensure calcium-dependent binding to membrane surfaces.
- The now mature and activated protein S will circulate in the blood in two states:
- Free protein S
- This form constitutes 30 to 40 percent of the total protein S in the body.
- It is the only form that will take part in the coagulation cascade.[8]
- C4b-bound protein S
- There is a high affinity interaction between protein S and C4b-binding protein.
- C4b-binding protein is a complement regulator; hence, it is responsible for controlling the activity of protein S.
- Around 70 percent of circulating protein S is in the bound form. [9]
- Free protein S
- The activated free protein S acts as a cofactor to activated protein C, and with the help of phospholipids and Ca2+, it inactivates procoagulant factor Va and factor VIIIa thereby reducing thrombin formation.[7]
- Protein S deficiency is a hereditary disease that results from mutations in the PROS1 gene, located on chromosome 3.
- This disease usually occurs due to heterozygous gene mutations in the PROS1 gene; however, rare cases of homozygous protein S deficiencies have been reported.
- Although another gene, PROS2, has been isolated on the same chromosome 3, it does not seem to have any relevance and has since been classified as a pseudogene.[10][11]
Causes
- In addition to the common hereditary form of protein S deficiency, there are rare circumstances in which acquired causes can result in diminished protein S levels:
Differentiating Protein S deficiency from Other Diseases
Protein S deficiency must be differentiated from other diseases that cause symptoms of DVT and pulmonary embolism such as:
- Factor V Leiden mutation
- Antithrombin III deficiency
- Protein C deficiency
- Prothrombin gene mutation
- Disseminated intravascular coagulation (DIC)
- Antiphospholipid antibody syndrome
For more information on differentiating protein S deficiency, click here.
Epidemiology and Demographics
- The prevalence of protein S deficiency in the general population is unknown.
- However, its prevalence in individuals with a history of venous thromboembolism is approximately 900 per 100,000 individuals worldwide. [18]
Age
- Patients of all age groups may be diagnosed with protein S deficiency.
- It is; however, more commonly observed among patients younger than 40 to 50 years old.
Gender
- There is no difference in the prevalence of the disease between men and women.
Race
- Protein S deficiency usually affects individuals of the Asian race.
- Caucasian individuals are less likely to develop protein S deficiency.
Risk Factors
- There are no established risk factors for protein S deficiency.
- First degree relatives pose the highest risk of developing protein S deficiency.
Screening
There is insufficient evidence to recommend routine screening for [disease/malignancy].
OR
According to the [guideline name], screening for [disease name] is not recommended.
OR
According to the [guideline name], screening for [disease name] by [test 1] is recommended every [duration] among patients with [condition 1], [condition 2], and [condition 3].
Natural History, Complications, and Prognosis
If left untreated, [#]% of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
OR
Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
OR
Prognosis is generally excellent/good/poor, and the 1/5/10-year mortality/survival rate of patients with [disease name] is approximately [#]%.
Diagnosis
Diagnostic Study of Choice
The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met: [criterion 1], [criterion 2], [criterion 3], and [criterion 4].
OR
The diagnosis of [disease name] is based on the [criteria name] criteria, which include [criterion 1], [criterion 2], and [criterion 3].
OR
The diagnosis of [disease name] is based on the [definition name] definition, which includes [criterion 1], [criterion 2], and [criterion 3].
OR
There are no established criteria for the diagnosis of [disease name].
History and Symptoms
The majority of patients with [disease name] are asymptomatic.
OR
The hallmark of [disease name] is [finding]. A positive history of [finding 1] and [finding 2] is suggestive of [disease name]. The most common symptoms of [disease name] include [symptom 1], [symptom 2], and [symptom 3]. Common symptoms of [disease] include [symptom 1], [symptom 2], and [symptom 3]. Less common symptoms of [disease name] include [symptom 1], [symptom 2], and [symptom 3].
Physical Examination
Patients with [disease name] usually appear [general appearance]. Physical examination of patients with [disease name] is usually remarkable for [finding 1], [finding 2], and [finding 3].
OR
Common physical examination findings of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
The presence of [finding(s)] on physical examination is diagnostic of [disease name].
OR
The presence of [finding(s)] on physical examination is highly suggestive of [disease name].
Laboratory Findings
An elevated/reduced concentration of serum/blood/urinary/CSF/other [lab test] is diagnostic of [disease name].
OR
Laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3].
OR
[Test] is usually normal among patients with [disease name].
OR
Some patients with [disease name] may have elevated/reduced concentration of [test], which is usually suggestive of [progression/complication].
OR
There are no diagnostic laboratory findings associated with [disease name].
Electrocardiogram
There are no ECG findings associated with [disease name].
OR
An ECG may be helpful in the diagnosis of [disease name]. Findings on an ECG suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
X-ray
There are no x-ray findings associated with [disease name].
OR
An x-ray may be helpful in the diagnosis of [disease name]. Findings on an x-ray suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no x-ray findings associated with [disease name]. However, an x-ray may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
Echocardiography or Ultrasound
There are no echocardiography/ultrasound findings associated with [disease name].
OR
Echocardiography/ultrasound may be helpful in the diagnosis of [disease name]. Findings on an echocardiography/ultrasound suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no echocardiography/ultrasound findings associated with [disease name]. However, an echocardiography/ultrasound may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
CT scan
There are no CT scan findings associated with [disease name].
OR
[Location] CT scan may be helpful in the diagnosis of [disease name]. Findings on CT scan suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no CT scan findings associated with [disease name]. However, a CT scan may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
MRI
There are no MRI findings associated with [disease name].
OR
[Location] MRI may be helpful in the diagnosis of [disease name]. Findings on MRI suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no MRI findings associated with [disease name]. However, a MRI may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
Other Imaging Findings
There are no other imaging findings associated with [disease name].
OR
[Imaging modality] may be helpful in the diagnosis of [disease name]. Findings on an [imaging modality] suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
Other Diagnostic Studies
There are no other diagnostic studies associated with [disease name].
OR
[Diagnostic study] may be helpful in the diagnosis of [disease name]. Findings suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
Other diagnostic studies for [disease name] include [diagnostic study 1], which demonstrates [finding 1], [finding 2], and [finding 3], and [diagnostic study 2], which demonstrates [finding 1], [finding 2], and [finding 3].
Treatment
Medical Therapy
There is no treatment for [disease name]; the mainstay of therapy is supportive care.
OR
Supportive therapy for [disease name] includes [therapy 1], [therapy 2], and [therapy 3].
OR
The majority of cases of [disease name] are self-limited and require only supportive care.
OR
[Disease name] is a medical emergency and requires prompt treatment.
OR
The mainstay of treatment for [disease name] is [therapy].
OR The optimal therapy for [malignancy name] depends on the stage at diagnosis.
OR
[Therapy] is recommended among all patients who develop [disease name].
OR
Pharmacologic medical therapy is recommended among patients with [disease subclass 1], [disease subclass 2], and [disease subclass 3].
OR
Pharmacologic medical therapies for [disease name] include (either) [therapy 1], [therapy 2], and/or [therapy 3].
OR
Empiric therapy for [disease name] depends on [disease factor 1] and [disease factor 2].
OR
Patients with [disease subclass 1] are treated with [therapy 1], whereas patients with [disease subclass 2] are treated with [therapy 2].
Surgery
Surgical intervention is not recommended for the management of [disease name].
OR
Surgery is not the first-line treatment option for patients with [disease name]. Surgery is usually reserved for patients with either [indication 1], [indication 2], and [indication 3]
OR
The mainstay of treatment for [disease name] is medical therapy. Surgery is usually reserved for patients with either [indication 1], [indication 2], and/or [indication 3].
OR
The feasibility of surgery depends on the stage of [malignancy] at diagnosis.
OR
Surgery is the mainstay of treatment for [disease or malignancy].
Primary Prevention
There are no established measures for the primary prevention of [disease name].
OR
There are no available vaccines against [disease name].
OR
Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3].
OR
[Vaccine name] vaccine is recommended for [patient population] to prevent [disease name]. Other primary prevention strategies include [strategy 1], [strategy 2], and [strategy 3].
Secondary Prevention
There are no established measures for the secondary prevention of [disease name].
References
- ↑ Comp PC, Nixon RR, Cooper MR, Esmon CT (1984). "Familial protein S deficiency is associated with recurrent thrombosis". J Clin Invest. 74 (6): 2082–8. doi:10.1172/JCI111632. PMC 425398. PMID 6239877.
- ↑ Gandrille S, Borgel D, Sala N, Espinosa-Parrilla Y, Simmonds R, Rezende S; et al. (2000). "Protein S deficiency: a database of mutations--summary of the first update". Thromb Haemost. 84 (5): 918. PMID 11127877.
- ↑ Schwarz HP, Fischer M, Hopmeier P, Batard MA, Griffin JH (1984). "Plasma protein S deficiency in familial thrombotic disease". Blood. 64 (6): 1297–300. PMID 6238642.
- ↑ Simmonds RE, Ireland H, Kunz G, Lane DA (1996). "Identification of 19 protein S gene mutations in patients with phenotypic protein S deficiency and thrombosis. Protein S Study Group". Blood. 88 (11): 4195–204. PMID 8943854.
- ↑ Gandrille S, Borgel D, Eschwege-Gufflet V, Aillaud M, Dreyfus M, Matheron C; et al. (1995). "Identification of 15 different candidate causal point mutations and three polymorphisms in 19 patients with protein S deficiency using a scanning method for the analysis of the protein S active gene". Blood. 85 (1): 130–8. PMID 7803790.
- ↑ "Protein C - Wikipedia".
- ↑ 7.0 7.1 Esmon CT (1992). "Protein S and protein C Biochemistry, physiology, and clinical manifestation of deficiencies". Trends Cardiovasc Med. 2 (6): 214–9. doi:10.1016/1050-1738(92)90027-P. PMID 21239244.
- ↑ Rezende SM, Simmonds RE, Lane DA (2004). "Coagulation, inflammation, and apoptosis: different roles for protein S and the protein S-C4b binding protein complex". Blood. 103 (4): 1192–201. doi:10.1182/blood-2003-05-1551. PMID 12907438.
- ↑ Dahlbäck B (2011). "C4b-binding protein: a forgotten factor in thrombosis and hemostasis". Semin Thromb Hemost. 37 (4): 355–61. doi:10.1055/s-0031-1276584. PMID 21805441.
- ↑ Ploos van Amstel JK, van der Zanden AL, Bakker E, Reitsma PH, Bertina RM (1987). "Two genes homologous with human protein S cDNA are located on chromosome 3". Thromb Haemost. 58 (4): 982–7. PMID 2895503.
- ↑ Schmidel DK, Tatro AV, Phelps LG, Tomczak JA, Long GL (1990). "Organization of the human protein S genes". Biochemistry. 29 (34): 7845–52. PMID 2148110.
- ↑ Comp PC, Thurnau GR, Welsh J, Esmon CT (1986). "Functional and immunologic protein S levels are decreased during pregnancy". Blood. 68 (4): 881–5. PMID 2944555.
- ↑ Comp PC, Doray D, Patton D, Esmon CT (1986). "An abnormal plasma distribution of protein S occurs in functional protein S deficiency". Blood. 67 (2): 504–8. PMID 2935211.
- ↑ Matsuzaka T, Tanaka H, Fukuda M, Aoki M, Tsuji Y, Kondoh H (1993). "Relationship between vitamin K dependent coagulation factors and anticoagulants (protein C and protein S) in neonatal vitamin K deficiency". Arch Dis Child. 68 (3 Spec No): 297–302. PMC 1590375. PMID 8466266.
- ↑ Gilabert J, Fernandez JA, España F, Aznar J, Estelles A (1988). "Physiological coagulation inhibitors (protein S, protein C and antithrombin III) in severe preeclamptic states and in users of oral contraceptives". Thromb Res. 49 (3): 319–29. PMID 2966452.
- ↑ Heeb MJ, Mosher D, Griffin JH (1989). "Activation and complexation of protein C and cleavage and decrease of protein S in plasma of patients with intravascular coagulation". Blood. 73 (2): 455–61. PMID 2521800.
- ↑ Vigano-D'Angelo S, D'Angelo A, Kaufman CE, Sholer C, Esmon CT, Comp PC (1987). "Protein S deficiency occurs in the nephrotic syndrome". Ann Intern Med. 107 (1): 42–7. PMID 2954500.
- ↑ Pintao MC, Ribeiro DD, Bezemer ID, Garcia AA, de Visser MC, Doggen CJ; et al. (2013). "Protein S levels and the risk of venous thrombosis: results from the MEGA case-control study". Blood. 122 (18): 3210–9. doi:10.1182/blood-2013-04-499335. PMID 24014240.