Sandbox:Cherry
Cherry
Physical Examination
| RT-8OzD9j00}} | -fTGzcsygBI}} |


 |
 |

Source:Wikimedia commons[1]

Source:Wikimedia commons

Source:Wikimedia commons
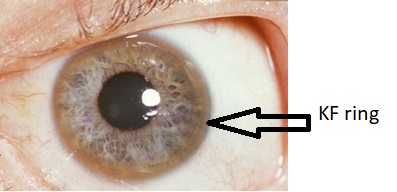
Eyes
Abdomen
{{#ev:youtube|CHUBTgrU3Oc}}

{{#ev:youtube|Or65nOrcz1A}}
Physical Examination
- Physical examination of patients with cirrhosis is usually remarkable for: jaundice, spider angiomata, ascites, asterixis, spleenomegaly and palmar erythema.
Appearance of the Patient
- Patients with cirrhosis usually appear weak due to constitutional symptoms such as weight loss, anorexia and muscle atrophy. Yellowish discoloration of skin and abdominal distension may also be present due to ascites.
- Normal/low blood pressure with normal pulse pressure.
Skin
- Jaundice : yellow discoloration of the skin, eyes, and mucus membranes due to increased bilirubin (at least 2-3 mg/dL or 30 mmol/L). Urine may also appear dark.
- Pallor
- Bruises
- Palmar erythema on the thenar and hypothenar eminences, due to altered sex hormone metabolism.
- Spider angiomata: Increased estradiol levels lead to the formation of vascular lesions consisting of central arterioles surrounded by smaller vessels [2]
- Telangiectasias or spider veins: small dilated blood vessels near the surface of the skin.
HEENT
- Abnormalities of the head/hair may include thinning of hair on the scalp due to hyperestrogenism
- Kayser-Fleischer rings : dark rings that appear to encircle the iris of the eye in patients with Wilson's disease.
- Parotid gland enlargement
- Fetor hepaticus: severe portal-systemic shunting leads to increased levels of dimethyl sulfide leads to a sweet pungent smell in the breath
Abdomen
- Inspection:
- Palpation:
- Fluid wave
- Hepatomegaly may be present in initial stages. The liver may also be normal or shrunken.
- Spleenomegaly may be present in patients with cirrhosis from nonalcoholic etiologies, due to portal hypertension
- Percussion:
- Flank dullness may be present due to ascites (needs approximately 1500ml for detection)
- Auscultation:
- Cruveilhier-Baumgarten murmur: venous hum that may be present in patients with portal hypertension.
- Mechanism: due to collateral connections between remnant of the umbilical vein and the portal system
- Location: Epigastrium
- Exacerbating factors: Valsalva maneuver
- Diminished by: application of pressure on the skin above the umbilicus
- Cruveilhier-Baumgarten murmur: venous hum that may be present in patients with portal hypertension.
Genitourinary
- Testicular atrophy
- Inversion of the normal male pubic hair pattern
Neuromuscular
- Hepatic encephalopathy may have signs of:
- Alteration of mental status
- Confusion
- Coma
- Asterixis (bilateral but asynchronous flapping motions of outstretched, dorsiflexed hands) is seen in patients with hepatic encephalopathy.
Extremities
- edema of the lower extremities
- Muscle atrophy
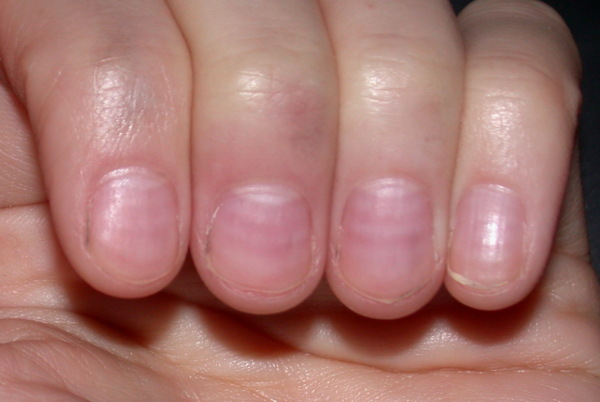
- Nail changes:
- Muehrcke nails: paired horizontal white bands separated by normal color due to hypoalbuminemia
- Terry nails: the proximal two-thirds of the nail plate appears white, whereas the distal one-third is red due to hypoalbuminemia
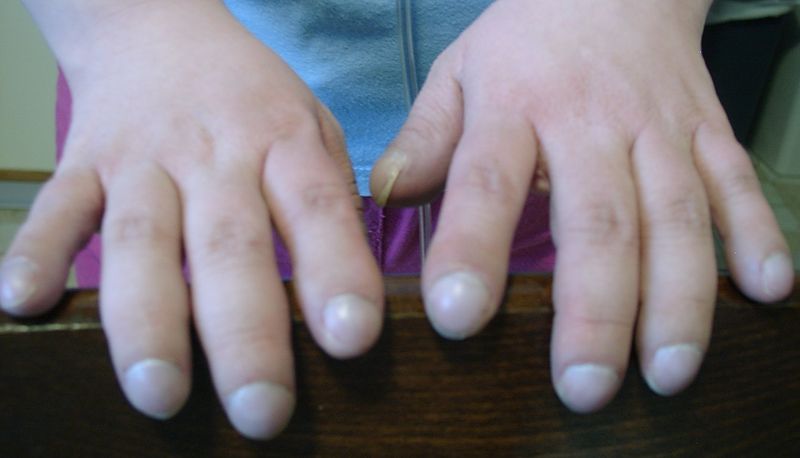
- Clubbing: the angle between the nail plate and proximal nail fold is greater than 180 degrees
- Severe clubbing:
- "Drum stick" appearance of distal fingers
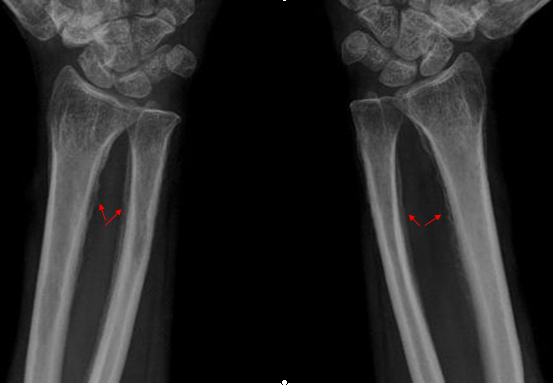
- Hypertrophic osteoarthropathy: chronic proliferative periostitis of the long bones
- Dupuytren's contracture may cause flexion deformities of the fingers: This occurs due to shortening and thickening of the palmar fascia, due to collagen deposition and fibroblastic proliferation.
- Asterixis in cases with hepatic encephalopathy
Chest findings
- Gynecomastia: due to increased estradiol levels
- Loss of chest or axillary hair
Other findings
History
Psychosocial history
- Past history of abuse
Past Medical history
- History of
Menstrual history
- History of
Family history
- Family history of:
Medication history
- History of medication use should be obtained as many drugs such as opioids cause constipation as a side effect.
Causes
| Drugs and Toxins | Infections | Autoimmune | Metabolic | Biliary obstruction(Secondary bilary cirrhosis) | Vascular | Miscellaneous |
|---|---|---|---|---|---|---|
| Alcohol | Hepatitis B | Primary Biliary Cirrhosis | Wilson's disease | Cystic fibrosis | Chronic RHF | Sarcoidosis |
| Methotrexate | Hepatitis C | Autoimmune hepatitis | Hemochromatosis | Biliary atresia | Budd-Chiari syndrome | Intestinal
bypass operations for obesity |
| Isoniazid | Schistosoma japonicum | Primary Sclerosing Cholangitis | Alpha-1 antitrypsin deficiency | Bile duct strictures | Veno-occlusive disease | Cryptogenic: unknown |
| Methyldopa | Porphyria | Gallstones | ||||
| Glycogen storage diseases (such as Galactosaemia, Abetalipoproteinaemia) |
Cirrhosis
Pathophysiology [3][4][5][6][7][8]
- When an injured issue is replaced by a collagenous scar, it is termed as fibrosis.
- When fibrosis of the liver reaches an advanced stage where distortion of the hepatic vasculature also occurs, it is termed as cirrhosis of the liver.
- The cellular mechanisms responsible for cirrhosis are similar regardless of the type of initial insult and site of injury within the liver lobule.
- Viral hepatitis involves the periportal region, whereas involvement in alcoholic liver disease is largely pericentral.
- If the damage progresses, panlobular cirrhosis may result.
- Cirrhosis involves the following steps: [9]
- Inflammation
- Hepatic stellate cell activation
- Angiogenesis
- Fibrogenesis
- Kupffer cells are hepatic macrophages responsible for Hepatic Stellate cell activation during injury.
- The hepatic stellate cell (also known as the perisinusoidal cell or Ito cell) plays a key role in the pathogenesis of liver fibrosis/cirrhosis.
- Hepatic stellate cells(HSC) are usually located in the subendothelial space of Disse and become activated to a myofibroblast-like phenotype in areas of liver injury.
- Collagen and non collagenous matrix proteins responsible for fibrosis are produced by the activated Hepatic Stellate Cells(HSC).
- Hepatocyte damage causes the release of lipid peroxidases from injured cell membranes leading to necrosis of parenchymal cells.
- Activated HSC produce numerous cytokines and their receptors, such as PDGF and TGF-f31 which are responsible for fibrogenesis.
- The matrix formed due to HSC activation is deposited in the space of Disse and leads to loss of fenestrations of endothelial cells, which is a process called capillarization.
- Cirrhosis leads to hepatic microvascular changes characterised by [10]
- formation of intra hepatic shunts (due to angiogenesis and loss of parenchymal cells)
- hepatic endothelial dysfunction
- The endothelial dysfunction is characterised by [11]
- insufficient release of vasodilators, such as nitric oxide due to oxidative stress
- increased production of vasoconstrictors (mainly adrenergic stimulation and activation of endothelins and RAAS)
- Fibrosis eventually leads to formation of septae that grossly distort the liver architecture which includes both the liver parenchyma and the vasculature. A cirrhotic liver compromises hepatic sinusoidal exchange by shunting arterial and portal blood directly into the central veins (hepatic outflow). Vascularized fibrous septa connect central veins with portal tracts leading to islands of hepatocytes surrounded by fibrous bands without central veins.[12][13][14]
- The formation of fibrotic bands is accompanied by regenerative nodule formation in the hepatic parenchyma.
- Advancement of cirrhosis may lead to parenchymal dysfunction and development of portal hypertension.
- Portal HTN results from the combination of the following:
- Structural disturbances associated with advanced liver disease account for 70% of total hepatic vascular resistance.
- Functional abnormalities such as endothelial dysfunction and increased hepatic vascular tone account for 30% of total hepatic vascular resistance.
Pathogenesis of Cirrhosis due to Alcohol:
- More than 66 percent of all American adults consume alcohol.
- Cirrhosis due to alcohol accounts for approximately forty percent of mortality rates due to cirrhosis.
- Mechanisms of alcohol-induced damage include:
- Impaired protein synthesis, secretion, glycosylation
- Ethanol intake leads to elevated accumulation of intracellular triglycerides by:
- Lipoprotein secretion
- Decreased fatty acid oxidation
- Increased fatty acid uptake
- Alcohol is converted by Alcohol dehydrogenase to acetaldehyde.
- Due to the high reactivity of acetaldehyde, it forms acetaldehyde-protein adducts which cause damage to cells by:
- Trafficking of hepatic proteins
- Interrupting microtubule formation
- Interfering with enzyme activities
- Damage of hepatocytes leads to the formation of reactive oxygen species that activate Kupffer cells.[8]
- Kupffer cell activation leads to the production of profibrogenic cytokines that stimulates stellate cells.
- Stellate cell activation leads to the production of extracellular matrix and collagen.
- Portal triads develop connections with central veins due to connective tissue formation in pericentral and periportal zones, leading to the formation of regenerative nodules.
- Shrinkage of the liver occurs over years due to repeated insults that lead to:
- Loss of hepatocytes
- Increased production and deposition of collagen
Pathology
- There are four stages of Cirrhosis as it progresses:
- Chronic nonsuppurative destructive cholangitis - inflammation and necrosis of portal tracts with lymphocyte infiltration leading to the destruction of the bile ducts.
- Development of biliary stasis and fibrosis
- Periportal fibrosis progresses to bridging fibrosis
- Increased proliferation of smaller bile ductules leading to regenerative nodule formation.
Classification
|
Cirrhosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Sandbox:Cherry On the Web |
|
American Roentgen Ray Society Images of Sandbox:Cherry |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Charmaine Patel, M.D. [2]
Overview
Cirrhosis of the liver can be classified using two methods; classification based on etiology, and classification based on morphology. Currently, classifying cirrhosis based on morphology is not used, as it requires an invasive procedure to examine the gross appearance of the liver, and it provides little diagnostic value. Classifying cirrhosis according to etiology is a more accepted form of classification, as it can be attained through non-invasive laboratory testing, and has a higher diagnostic value.
Classification Based on Etiology
Cirrhosis can be classified by its etiology. This is the most widely accepted method of classification.
Alcoholic Cirrhosis
This is the most common cause of cirrhosis, and is caused by continuous and prolonged alcohol abuse. The American Academy of Family Physicians estimate that 60-70 percent of all cases of cirrhosis are a result of alcohol abuse.
Post-Necrotic Cirrhosis
This type of cirrhosis occurs after a massive event causes liver cell death. Viral hepatitis is the most common cause for this type of cirrhosis. Agents that are toxic to the liver can also cause this type of cirrhosis, as well as certain types of carcinomas.
Biliary Cirrhosis
This type of cirrhosis results from any diseases that cause biliary obstruction. There is usually a blockage in the bile duct and there may also be inflammation. The excess bile in the liver causes tissue destruction. It commonly results in jaundice.
Cardiac Cirrhosis
This type of cirrhosis is caused by congestive heart failure causing poor circulation of oxygenated blood to the liver. This results in liver cell death, and the subsequent replacement of dead cells by fibrous tissue.
Genetic Disorder
This is when the cirrhosis is caused by a genetic disorder such as hemochromatosis, Wilson's disease, or alpha-1 antitrypsin deficiency.
Malnutrition
This category contains cirrhosis caused by various forms of malnutrition, particularly chronic starvation.
Classification Based on Morphology
Cirrhosis has historically been classified upon the nodular morphology that is seen on upon the gross appearance of the liver. Accurate assessment of the liver morphology can only be obtained through surgery, biopsy, or autopsy, therefore more recently, more non-invasive means of classifying and determining the causes of cirrhosis are used.
| Micronodular | Macronodular | Mixed |
|---|---|---|
| Micronodular cirrhosis is characterized by nodules that are less than 3mm in diameter | Macronodular cirrhosis is characterized by nodules that are more than 3mm in diameter | Micronodular cirrhosis can often progress into macronodular cirrhosis. During this transformation, a mixed form of cirrhosis may be seen.[15] |
Causes:
|
Causes:
|
Mixed nodular cirrhosis is also seen in Indian childhood cirrhosis. [16] |
References
- ↑ "Category:Histopathology of cirrhosis - Wikimedia Commons".
- ↑ Li CP, Lee FY, Hwang SJ; et al. (1999). "Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function". Scand. J. Gastroenterol. 34 (5): 520–3. PMID 10423070.
- ↑ Arthur MJ, Iredale JP (1994). "Hepatic lipocytes, TIMP-1 and liver fibrosis". J R Coll Physicians Lond. 28 (3): 200–8. PMID 7932316.
- ↑ Friedman SL (1993). "Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies". N. Engl. J. Med. 328 (25): 1828–35. doi:10.1056/NEJM199306243282508. PMID 8502273.
- ↑ Iredale JP (1996). "Matrix turnover in fibrogenesis". Hepatogastroenterology. 43 (7): 56–71. PMID 8682489.
- ↑ Gressner AM (1994). "Perisinusoidal lipocytes and fibrogenesis". Gut. 35 (10): 1331–3. PMC 1374996. PMID 7959178.
- ↑ Iredale JP (2007). "Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ". J. Clin. Invest. 117 (3): 539–48. doi:10.1172/JCI30542. PMC 1804370. PMID 17332881.
- ↑ 8.0 8.1 Arthur MJ (2002). "Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C". Gastroenterology. 122 (5): 1525–8. PMID 11984538.
- ↑ Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G (1995). "Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension". Hepatology. 21 (5): 1238–47. PMID 7737629.
- ↑ Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J (2009). "Angiogenesis in liver disease". J. Hepatol. 50 (3): 604–20. doi:10.1016/j.jhep.2008.12.011. PMID 19157625.
- ↑ García-Pagán JC, Gracia-Sancho J, Bosch J (2012). "Functional aspects on the pathophysiology of portal hypertension in cirrhosis". J. Hepatol. 57 (2): 458–61. doi:10.1016/j.jhep.2012.03.007. PMID 22504334.
- ↑ Schuppan D, Afdhal NH (2008). "Liver cirrhosis". Lancet. 371 (9615): 838–51. doi:10.1016/S0140-6736(08)60383-9. PMC 2271178. PMID 18328931.
- ↑ Desmet VJ, Roskams T (2004). "Cirrhosis reversal: a duel between dogma and myth". J. Hepatol. 40 (5): 860–7. doi:10.1016/j.jhep.2004.03.007. PMID 15094237.
- ↑ Wanless IR, Nakashima E, Sherman M (2000). "Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis". Arch. Pathol. Lab. Med. 124 (11): 1599–607. doi:10.1043/0003-9985(2000)124<1599:ROHC>2.0.CO;2. PMID 11079009.
- ↑ Fauerholdt L, Schlichting P, Christensen E, Poulsen H, Tygstrup N, Juhl E (1983). "Conversion of micronodular cirrhosis into macronodular cirrhosis". Hepatology. 3 (6): 928–31. PMID 6629323.
- ↑ Nayak NC, Ramalingaswami V (1975). "Indian childhood cirrhosis". Clin Gastroenterol. 4 (2): 333–49. PMID 47794.
REFERENCES