Sacubitril and Valsartan: Difference between revisions
No edit summary |
No edit summary |
||
| Line 104: | Line 104: | ||
***ENTRESTO is teratogenic based on a low incidence of fetal hydrocephaly, associated with maternally toxic doses, which was observed in rabbits at an ENTRESTO dose of ≥ 5 mg sacubitril/5 mg valsartan/kg/day. The adverse embryo-fetal effects of ENTRESTO are attributed to the angiotensin receptor antagonist activity. | ***ENTRESTO is teratogenic based on a low incidence of fetal hydrocephaly, associated with maternally toxic doses, which was observed in rabbits at an ENTRESTO dose of ≥ 5 mg sacubitril/5 mg valsartan/kg/day. The adverse embryo-fetal effects of ENTRESTO are attributed to the angiotensin receptor antagonist activity. | ||
***Pre- and postnatal development studies in rats at sacubitril doses up to 750 mg/kg/day (4.5-fold the MRHD on the basis of LBQ657 AUC) and valsartan at doses up to 600 mg/kg/day (0.86-fold the MRHD on the basis of AUC) indicate that treatment with ENTRESTO during organogenesis, gestation and lactation may affect pup development and survival. | ***Pre- and postnatal development studies in rats at sacubitril doses up to 750 mg/kg/day (4.5-fold the MRHD on the basis of LBQ657 AUC) and valsartan at doses up to 600 mg/kg/day (0.86-fold the MRHD on the basis of AUC) indicate that treatment with ENTRESTO during organogenesis, gestation and lactation may affect pup development and survival. | ||
|useInNursing=*Risk Summary | |||
**There is no information regarding the presence of sacubitril/valsartan in human milk, the effects on the breastfed infant, or the effects on milk production. Sacubitril/valsartan is present in rat milk. Because of the potential for serious adverse reactions in breastfed infants from exposure to sacubitril/valsartan, advise a nursing woman that breastfeeding is not recommended during treatment with ENTRESTO. | |||
*Data | |||
**Following an oral dose (15 mg sacubitril/15 mg valsartan/kg) of [14C] ENTRESTO to lactating rats, transfer of LBQ657 into milk was observed. After a single oral administration of 3 mg/kg [14C] valsartan to lactating rats, transfer of valsartan into milk was observed. | |||
|useInPed=Safety and effectiveness in pediatric patients have not been established. | |||
|useInGeri=No relevant pharmacokinetic differences have been observed in elderly (≥65 years) or very elderly (≥75 years) patients compared to the overall population. | |||
|alcohol=Alcohol-Sacubitril and Valsartan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Sacubitril and Valsartan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 20:56, 24 July 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: AKT
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
|
Overview
Sacubitril and Valsartan is a combination of a neprilysin inhibitor and an angiotensin II receptor blocker that is FDA approved for the prevention of of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypotension, increased serum potassium, hyperkalemia, and increased serum creatinine.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
ENTRESTO is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction.
Dosing Information
- ENTRESTO is contraindicated with concomitant use of an angiotensin-converting enzyme (ACE) inhibitor. If switching from an ACE inhibitor to ENTRESTO allow a washout period of 36 hours between administration of the two drugs.
- The recommended starting dose of ENTRESTO is 49/51 mg twice-daily.
- Double the dose of ENTRESTO after 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.
- Dose Adjustment for Patients Not Taking an ACE inhibitor or ARB or Previously Taking Low Doses of These Agents
- A starting dose of 24/26 mg twice-daily is recommended for patients not currently taking an ACE inhibitor or an angiotensin II receptor blocker (ARB) and for patients previously taking low doses of these agents. Double the dose of ENTRESTO every 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.
- Dose Adjustment for Severe Renal Impairment
- A starting dose of 24/26 mg twice-daily is recommended for patients with severe renal impairment (eGFR <30 mL/min/1.73 m2). Double the dose of ENTRESTO every 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.
- No starting dose adjustment is needed for mild or moderate renal impairment.
- Dose Adjustment for Hepatic Impairment
- A starting dose of 24/26 mg twice-daily is recommended for patients with moderate hepatic impairment (Child-Pugh B classification). Double the dose of ENTRESTO every 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.
- No starting dose adjustment is needed for mild hepatic impairment.
- Use in patients with severe hepatic impairment is not recommended.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sacubitril and Valsartan in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sacubitril and Valsartan in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Sacubitril and Valsartan FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sacubitril and Valsartan in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sacubitril and Valsartan in pediatric patients.
Contraindications
ENTRESTO is contraindicated:
- In patients with hypersensitivity to any component.
- In patients with a history of angioedema related to previous ACE inhibitor or ARB therapy.
- With concomitant use of ACE inhibitors. Do not administer within 36 hours of switching from or to an ACE inhibitor.
- With concomitant use of aliskiren in patients with diabetes.
Warnings
|
FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
|
- Fetal Toxicity
- ENTRESTO can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death.
- When pregnancy is detected, consider alternative drug treatment and discontinue ENTRESTO. However, if there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system, and if the drug is considered lifesaving for the mother, advise a pregnant woman of the potential risk to the fetus.
- Angioedema
- ENTRESTO may cause angioedema. In the double-blind period of PARADIGM-HF, 0.5% of patients treated with ENTRESTO and 0.2% of patients treated with enalapril had angioedema.
- If angioedema occurs, discontinue ENTRESTO immediately, provide appropriate therapy, and monitor for airway compromise. ENTRESTO must not be re-administered. In cases of confirmed angioedema where swelling has been confined to the face and lips, the condition has generally resolved without treatment, although antihistamines have been useful in relieving symptoms.
- Angioedema associated with laryngeal edema may be fatal. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, administer appropriate therapy, e.g., subcutaneous epinephrine/adrenaline solution 1:1000 (0.3 mL to 0.5 mL) and take measures necessary to ensure maintenance of a patent airway.
- ENTRESTO has been associated with a higher rate of angioedema in Black than in non-Black patients.
- Patients with a prior history of angioedema may be at increased risk of angioedema with ENTRESTO. ENTRESTO should not be used in patients with a known history of angioedema related to previous ACE inhibitor or ARB therapy.
- Hypotension
- ENTRESTO lowers blood pressure and may cause symptomatic hypotension. Patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients (e.g., those being treated with high doses of diuretics), are at greater risk. In the double-blind period of PARADIGM-HF, 18% of patients treated with ENTRESTO and 12% of patients treated with enalapril reported hypotension as an adverse event, with hypotension reported as a serious adverse event in approximately 1.5% of patients in both treatment arms.
- Correct volume or salt depletion prior to administration of ENTRESTO or start at a lower dose. If hypotension occurs, consider dose adjustment of diuretics, concomitant antihypertensive drugs, and treatment of other causes of hypotension (e.g., hypovolemia). If hypotension persists despite such measures, reduce the dosage or temporarily discontinue ENTRESTO. Permanent discontinuation of therapy is usually not required.
- Impaired Renal Function
- As a consequence of inhibiting the renin-angiotensin-aldosterone system (RAAS), decreases in renal function may be anticipated in susceptible individuals treated with ENTRESTO. In the double-blind period of PARADIGM-HF, 5% of patients in both the ENTRESTO and enalapril groups reported renal failure as an adverse event.
- In patients whose renal function depends upon the activity of the renin-angiotensin-aldosterone system (e.g., patients with severe congestive heart failure), treatment with ACE inhibitors and angiotensin receptor antagonists has been associated with oliguria, progressive azotemia and, rarely, acute renal failure and death.
- Closely monitor serum creatinine, and down-titrate or interrupt ENTRESTO in patients who develop a clinically significant decrease in renal function.
- As with all drugs that affect the RAAS, ENTRESTO may increase blood urea and serum creatinine levels in patients with bilateral or unilateral renal artery stenosis. In patients with renal artery stenosis, monitor renal function.
- Hyperkalemia
- Through its actions on the RAAS, hyperkalemia may occur with ENTRESTO. In the double-blind period of PARADIGM-HF, 12% of patients treated with ENTRESTO and 14% of patients treated with enalapril reported hyperkalemia as an adverse event.
- Monitor serum potassium periodically and treat appropriately, especially in patients with risk factors for hyperkalemia such as severe renal impairment, diabetes, hypoaldosteronism, or a high potassium diet. Dosage reduction or interruption of ENTRESTO may be required.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the PARADIGM-HF trial, subjects were required to complete sequential enalapril and ENTRESTO run-in periods of (median) 15 and 29 days, respectively, prior to entering the randomized double-blind period comparing ENTRESTO and enalapril. During the enalapril run-in period, 1,102 patients (10.5%) were permanently discontinued from the study, 5.6% because of an adverse event, most commonly renal dysfunction (1.7%), hyperkalemia (1.7%) and hypotension (1.4%). During the ENTRESTO run-in period, an additional 10.4% of patients permanently discontinued treatment, 5.9% because of an adverse event, most commonly renal dysfunction (1.8%), hypotension (1.7%) and hyperkalemia (1.3%). Because of this run-in design, the adverse reaction rates described below are lower than expected in practice.
In the double-blind period, safety was evaluated in 4,203 patients treated with ENTRESTO and 4,229 treated with enalapril. In PARADIGM-HF, patients randomized to ENTRESTO received treatment for up to 4.3 years, with a median duration of exposure of 24 months; 3,271 patients were treated for more than one year. Discontinuation of therapy because of an adverse event during the double-blind period occurred in 450 (10.7%) of ENTRESTO treated patients and 516 (12.2%) of patients receiving enalapril.
Adverse reactions occurring at an incidence of ≥5% in patients who were treated with ENTRESTO in the double-blind period are shown in Table 1.
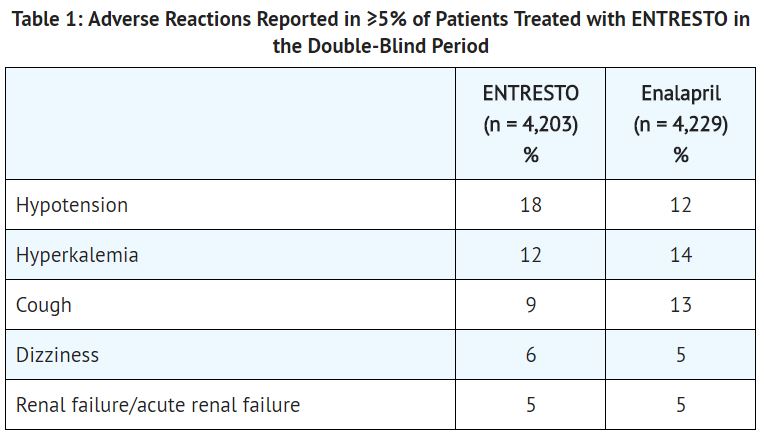
In the PARADIGM-HF trial, the incidence of angioedema was 0.1% in both the enalapril and ENTRESTO run-in periods. In the double-blind period, the incidence of angioedema was higher in patients treated with ENTRESTO than enalapril (0.5% and 0.2%, respectively). The incidence of angioedema in Black patients was 2.4% with ENTRESTO and 0.5% with enalapril.
Orthostasis was reported in 2.1% of patients treated with ENTRESTO compared to 1.1% of patients treated with enalapril during the double-blind period of PARADIGM-HF. Falls were reported in 1.9% of patients treated with ENTRESTO compared to 1.3% of patients treated with enalapril.
Laboratory Abnormalities:
- Hemoglobin and Hematocrit
- Decreases in hemoglobin/hematocrit of >20% were observed in approximately 5% of both ENTRESTO- and enalapril-treated patients in the double-blind period in PARADIGM-HF.
- Serum Creatinine
- Increases in serum creatinine of >50% were observed in 1.4% of patients in the enalapril run-in period and 2.2% of patients in the ENTRESTO run-in period. During the double-blind period, approximately 16% of both ENTRESTO- and enalapril-treated patients had increases in serum creatinine of >50%.
- Serum Potassium
- Potassium concentrations >5.5 mEq/L were observed in approximately 4% of patients in both the enalapril and ENTRESTO run-in periods. During the double-blind period, approximately 16% of both ENTRESTO- and enalapril-treated patients had potassium concentrations >5.5 mEq/L.
Postmarketing Experience
There is limited information regarding Sacubitril and Valsartan Postmarketing Experience in the drug label.
Drug Interactions
- Dual Blockade of the Renin-Angiotensin-Aldosterone System
- Concomitant use of ENTRESTO with an ACE inhibitor is contraindicated because of the increased risk of angioedema.
- Avoid use of ENTRESTO with an ARB, because ENTRESTO contains the angiotensin II receptor blocker valsartan.
- The concomitant use of ENTRESTO with aliskiren is contraindicated in patients with diabetes. Avoid use with aliskiren in patients with renal impairment (eGFR <60 mL/min/1.73 m2).
- Potassium-Sparing Diuretics
- As with other drugs that block angiotensin II or its effects, concomitant use of potassium-sparing diuretics (e.g., spironolactone, triamterene, amiloride), potassium supplements, or salt substitutes containing potassium may lead to increases in serum potassium.
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
- In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, concomitant use of NSAIDs, including COX-2 inhibitors, with ENTRESTO may result in worsening of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically.
- Lithium
- Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor antagonists. Monitor serum lithium levels during concomitant use with ENTRESTO.
Use in Specific Populations
Pregnancy
- Risk Summary
- ENTRESTO can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death.
- Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. In animal reproduction studies, ENTRESTO treatment during organogenesis resulted in increased embryo-fetal lethality in rats and rabbits and teratogenicity in rabbits.
- When pregnancy is detected, consider alternative drug treatment and discontinue ENTRESTO. However, if there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system, and if the drug is considered lifesaving for the mother, advise a pregnant woman of the potential risk to the fetus.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
- Clinical Considerations
- Fetal/Neonatal Adverse Reactions
- Oligohydramnios in pregnant women who use drugs affecting the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia, skeletal deformations, including skull hypoplasia, hypotension, and death.
- Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of gestation. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. If oligohydramnios is observed, consider alternative drug treatment.
- Closely observe neonates with histories of in utero exposure to ENTRESTO for hypotension, oliguria, and hyperkalemia. In neonates with a history of in utero exposure to ENTRESTO, if oliguria or hypotension occurs, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and replacing renal function.
- Fetal/Neonatal Adverse Reactions
- Data
- Animal data
- ENTRESTO treatment during organogenesis resulted in increased embryo-fetal lethality in rats at doses ≥ 49 mg sacubitril/51 mg valsartan/kg/day (≤ 0.14 [LBQ657, the active metabolite] and 1.5 [valsartan]-fold the maximum recommended human dose [MRHD] of 97/103 mg twice-daily on the basis of the area under the plasma drug concentration-time curve [AUC]) and rabbits at doses ≥ 5 mg sacubitril/5 mg valsartan/kg/day (4-fold and 0.06-fold the MRHD on the basis of valsartan and LBQ657 AUC, respectively).
- ENTRESTO is teratogenic based on a low incidence of fetal hydrocephaly, associated with maternally toxic doses, which was observed in rabbits at an ENTRESTO dose of ≥ 5 mg sacubitril/5 mg valsartan/kg/day. The adverse embryo-fetal effects of ENTRESTO are attributed to the angiotensin receptor antagonist activity.
- Pre- and postnatal development studies in rats at sacubitril doses up to 750 mg/kg/day (4.5-fold the MRHD on the basis of LBQ657 AUC) and valsartan at doses up to 600 mg/kg/day (0.86-fold the MRHD on the basis of AUC) indicate that treatment with ENTRESTO during organogenesis, gestation and lactation may affect pup development and survival.
- Animal data
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sacubitril and Valsartan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sacubitril and Valsartan during labor and delivery.
Nursing Mothers
- Risk Summary
- There is no information regarding the presence of sacubitril/valsartan in human milk, the effects on the breastfed infant, or the effects on milk production. Sacubitril/valsartan is present in rat milk. Because of the potential for serious adverse reactions in breastfed infants from exposure to sacubitril/valsartan, advise a nursing woman that breastfeeding is not recommended during treatment with ENTRESTO.
- Data
- Following an oral dose (15 mg sacubitril/15 mg valsartan/kg) of [14C] ENTRESTO to lactating rats, transfer of LBQ657 into milk was observed. After a single oral administration of 3 mg/kg [14C] valsartan to lactating rats, transfer of valsartan into milk was observed.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
No relevant pharmacokinetic differences have been observed in elderly (≥65 years) or very elderly (≥75 years) patients compared to the overall population.
Gender
There is no FDA guidance on the use of Sacubitril and Valsartan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sacubitril and Valsartan with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sacubitril and Valsartan in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sacubitril and Valsartan in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sacubitril and Valsartan in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sacubitril and Valsartan in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Sacubitril and Valsartan Administration in the drug label.
Monitoring
There is limited information regarding Sacubitril and Valsartan Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sacubitril and Valsartan and IV administrations.
Overdosage
There is limited information regarding Sacubitril and Valsartan overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Sacubitril and Valsartan Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Sacubitril and Valsartan Mechanism of Action in the drug label.
Structure
There is limited information regarding Sacubitril and Valsartan Structure in the drug label.
Pharmacodynamics
There is limited information regarding Sacubitril and Valsartan Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Sacubitril and Valsartan Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Sacubitril and Valsartan Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sacubitril and Valsartan Clinical Studies in the drug label.
How Supplied
There is limited information regarding Sacubitril and Valsartan How Supplied in the drug label.
Storage
There is limited information regarding Sacubitril and Valsartan Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sacubitril and Valsartan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sacubitril and Valsartan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sacubitril and Valsartan Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sacubitril and Valsartan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sacubitril and Valsartan Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sacubitril and Valsartan Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.