Rupatadine
 | |
| Clinical data | |
|---|---|
| Trade names | Rupafin, Alergoliber, Rinialer, Pafinur, Rupax, Ralif |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 98–99% |
| Metabolism | Hepatic, CYP-mediated |
| Elimination half-life | 5.9 hours |
| Excretion | 34.6% urine, 60.9% faeces |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
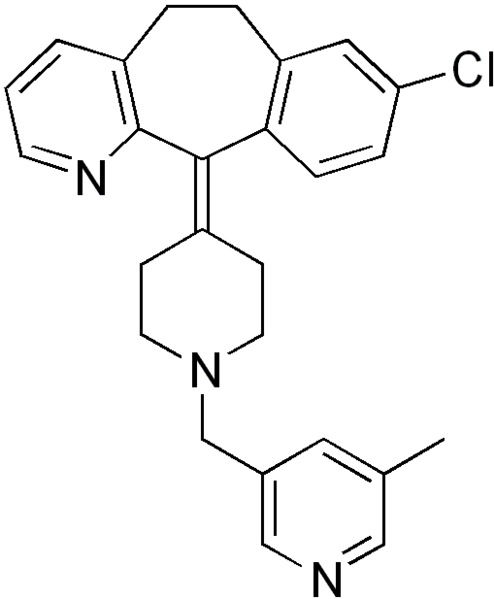
| Formula | C26H26ClN3 |
| Molar mass | 415.958 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Rupatadine |
|
Articles |
|---|
|
Most recent articles on Rupatadine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Rupatadine at Clinical Trials.gov Clinical Trials on Rupatadine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Rupatadine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Rupatadine Discussion groups on Rupatadine Patient Handouts on Rupatadine Directions to Hospitals Treating Rupatadine Risk calculators and risk factors for Rupatadine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Rupatadine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Rupatadine is a second generation antihistamine and PAF antagonist used to treat allergies. It was discovered and developed by J. Uriach y Cia, S. A.[1] and is marketed under several trade names such as Rupafin, Alergoliber, Rinialer, Pafinur, Rupax and Ralif.
Therapeutic indications approved
Rupatadine fumarate has been approved for the treatment of allergic rhinitis and chronic urticaria in adults and children over 12 years. The defined daily dose (DDD) is 10 mg orally.
Available form
Rupatadine is available as round, light salmon coloured tablets containing 10 mg of rupatadine (as fumarate) to be administered orally, once a day.
Side effects
Rupatadine is a non-sedating antihistamine. However, as in other non sedating second-generation antihistamines, the most common side effects in controlled clinical studies were somnolence, headaches and fatigue.
Mechanism of action
Rupatadine is a second generation, non-sedating, long-acting histamine antagonist with selective peripheral H1 receptor antagonist activity. It further blocks the receptors of the platelet-activating factor (PAF) according to in vitro and in vivo studies.[2]
Rupatadine possesses anti-allergic properties such as the inhibition of the degranulation of mast cells induced by immunological and non-immunological stimuli, and inhibition of the release of cytokines, particularly of the TNF in human mast cells and monocytes.[3]
Pharmacokinetics
Rupatadine has several active metabolites such as desloratadine, 3-hydroxydesloratadine, 6-hydroxydesloratadine and 5-hydroxydesloratadine.
History
Rupatadine discovery, pre-clinical and clinical development was performed by J. Uriach y Cia, S. A., a Spanish pharmaceutical company. It was launched in 2003 in Spain by J. Uriach y Cia, S. A., with the brand name of Rupafin. The registration of the product is approved in 23 countries from the EU, 8 Central American countries, Brazil, Argentina, Chile, Turkey and 14 African countries.
Efficacy in humans
The efficacy of rupatadine as treatment for allergic rhinitis (AR) and chronic idiopathic urticaria (CIU) has been investigated in adults and adolescents (aged over 12 years) in several controlled studies, showing a rapid onset of action and a good safety profile even in prolonged treatment periods of a year.[3][4][5]
References
- ↑ Patents: Template:Patent, Template:Patent, Template:Patent
- ↑ PMID 8996188 (PMID 8996188)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 3.0 3.1 PMID 17020424 (PMID 17020424)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 17335300 (PMID 17335300)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 18339040 (PMID 18339040)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
- Pages with script errors
- Pages with incomplete PMID references
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drug
- H1 receptor antagonists
- Pyridines
- Piperidines
- Organochlorides
- Benzocycloheptapyridines