Rubidium Rb 82
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: UNINTENDED STRONTIUM-82 (SR-82) AND STRONTIUM-85 (SR-85) RADIATION EXPOSURE:
See full prescribing information for complete Boxed Warning.
UNINTENDED STRONTIUM-82 (SR-82) AND STRONTIUM-85 (SR-85) RADIATION EXPOSURE:
Perform generator eluate tests: Record each generator eluate volume, including waste and test volumes, and keep a record of the cumulative eluate volume. Determine Rb-82, Sr-82, Sr-85 in the generator eluate: ●Once a day, prior to any drug administrations, and ●At additional daily tests after detection of an Alert Limit. Alert Limits are: ○14 L for the generator’s cumulative eluate volume, or ○An eluate Sr-82 level of 0.002 μCi/ mCi Rb-82, or ○An eluate Sr-85 level of 0.02 Sr-85 μCi/ mCi Rb-82. ○Perform the additional daily tests at time points determined by the day’s elution volume; tests are performed every 750 mL [see Dosage and Administration (2.5)]. Stop use of a generator at an Expiration Limit of: ○17 L for the generator’s cumulative eluate volume, or ○42 days post generator calibration date, or ○An eluate Sr-82 level of 0.01 μCi /mCi Rb-82, or ○An eluate Sr-85 level of 0.1 μCi /mCi Rb-82 |
Overview
Rubidium Rb 82 is a Diagnostic Agent that is FDA approved for the diagnosis of coronary artery disease. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Unintended radiation exposure.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- CardioGen-82 is a closed system used to produce rubidium Rb 82 chloride injection for intravenous administration. Rubidium Rb 82 chloride injection is indicated for Positron Emission Tomography (PET) imaging of the myocardium under rest or pharmacologic stress conditions to evaluate regional myocardial perfusion in adult patients with suspected or existing coronary artery disease.
Dosage
Infusion System
- Use CardioGen-82 only with an infusion system specifically designed for use with the generator and capable of accurate measurement and delivery of doses of rubidium Rb 82 chloride injection. Follow instructions in the Infusion System User’s Guide for the set up and intravenous infusion of rubidium Rb 82 chloride injection dose(s).
Rubidium Rb 82 Chloride Injection Dosage
- The recommended adult single dose of rubidium Rb 82 chloride injection is 1480 MBq (40 mCi) with a range of 1110-2220 MBq (30-60 mCi).
- Do not exceed a single dose of 2220 MBq (60 mCi).
- Use the lowest dose necessary to obtain adequate cardiac visualization consistent with the dosing goal of as low as reasonably achievable (ALARA).
- Individualize the dose by considering factors such as body size, and the imaging equipment and technique.
- Administer the single dose at 50 mL/minute through a catheter inserted into a large peripheral vein; do not to exceed a total infusion volume of 100 mL.
- Administer two separate single doses to complete rest and stress myocardial perfusion imaging as follows:
- Administer a single (“rest”) rubidium Rb-82 chloride dose;
- Start imaging 60-90 seconds after completion of the infusion of the rest dose and acquire images for 5 minutes; if a longer circulation time is anticipated (e.g., in a patient with severe left ventricular dysfunction), start imaging 120 seconds after the rest dose.
For stress imaging:
- Begin the study 10 minutes after completion of the resting dose infusion, to allow for sufficient Rb-82 decay;
- Administer a pharmacologic stress agent in accordance with its prescribing information;
- After an interval of 3 minutes, infuse a single (“stress”) rubidium Rb-82 chloride dose;
- Start imaging 60-90 seconds after completion of the stress Rb-82 chloride dose infusion and acquire images for 5 minutes; if a longer circulation time is anticipated start imaging 120 sec after the stress dose.
Drug Handling
- Limit the use of radiopharmaceuticals to physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
- Wear waterproof gloves and effective shielding when handling rubidium Rb-82 chloride injection and the infusion system.
- Observe aseptic techniques in all drug handling.
- Use only additive-free Sodium Chloride Injection USP to elute the generator.
- Visually inspect the drug for particulate matter and discoloration prior to administration whenever solution and container permit. Do not administer eluate from the generator if there is any evidence of foreign matter.
Directions for Eluting Rubidium Rb 82 Chloride Injection
- Allow at least 10 minutes between elutions for regeneration of Rb-82.
- Elute with additive-free Sodium Chloride Injection USP only. Additives (particularly calcium ions, to which strontium ions are chemically analogous), may cause the release of substantial amounts of Sr-82 and/or Sr-85 into the eluate regardless of the age or prior use of the generator.
- Discard the first 50 mL eluate each day the generator is first eluted. Employ proper safety precautions since the eluate contains radioactivity.
- Maintain an on-going record of all eluate volumes (washing, testing, dosing volumes), including a summary of the cumulative volume of eluate from the generator.
Eluate Testing Protocol
- Use additive-free sodium chloride injection USP for all elutions. Apply aseptic technique throughout.
- Before administering rubidium Rb 82 chloride injection to the first patient each day, perform the following test:
- Strontium Alert Limits and Mandatory Eluate Testing:
- Use an ionization chamber-type dose calibrator for eluate testing.
- Daily, before administering rubidium Rb 82 chloride injection to any patient, perform an eluate testing to determine Rb-82, Sr-82, and Sr-85 levels
- Perform additional daily eluate tests after detecting any of the following Alert Limits:
- 14 L total elution volume has passed through the generator column, or
- Sr-82 level reaches 0.002 µCi per mCi Rb-82, or
- Sr-85 level reaches 0.02 µCi per mCi Rb-82.
- Perform the additional daily eluate tests at time points determined by the day’s elution volume; tests are performed every 750 mL.
- For example, if an Alert Limit were reached and the clinical site eluted less than 750 mL from the generator during the day, then no additional eluate tests would have been performed that day.
- If the same clinical site the next day eluted 1,500 mL from the generator, then the site would have performed three tests that day: 1) the required daily test that precedes any patient dosing, 2) a test at the 750 mL elution point, and 3) a test at the 1,500 mL elution point.
- If a generator’s Alert Limit is reached, the clinical site performs the additional daily tests (at intervals of 750 mL) each subsequent day the generator is used. The additional tests are necessary to promptly detect excessive Sr-82 and/or Sr-85 in eluates.
Rubidium Eluate Level Testing:
- Set a dose calibrator for Rb-82 as recommended by the manufacturer or use the Co-60 setting and divide the reading obtained by 0.548. Obtain the reading from the instrument in millicuries.
- Elute the generator with 50 mL of Sodium Chloride Injection USP and discard the eluate (first elution).
- Allow at least 10 minutes for the regeneration of Rb-82, then elute the generator with 50 mL of Sodium Chloride Injection USP at a rate of 50 mL/min and collect the eluate in a stoppered glass vial (plastic containers are not suitable). Note the exact time of end of elution (E.O.E.).
- Using the dose calibrator, determine the activity of Rb-82 and note the time of the reading. Correct the reading for decay to the E.O.E. using the appropriate decay factor for Rb-82 (see Table 1).
- Note: If the reading is taken 2 1/2 minutes after end of elution, multiply the dose calibrator reading by 4 to correct for decay.
Strontium Eluate Level Testing
- Using the sample obtained for the Rb-82 activity determination, allow the sample to stand for at least one hour to allow for the complete decay of Rb-82.
- Measure the activity of the sample in a dose calibrator at the setting recommended by the manufacturer for Rb-82 and/or Sr-82. As an alternative, use the Co-60 setting and the reading obtained divided by 0.548. Set the instrument to read in microcuries and record the reading in the display.
- Calculate the ratio (R) of Sr-85/Sr-82 on the day (post-calibration) of the measurement using the ratio of Sr-85/Sr-82 on the day of calibration provided on the generator label and the Sr-85/Sr-82 Ratio Factor from Table 2. Determine R using the following equation:
R = [Sr-85] / [Sr-82] on calibration date X Ratio Factor on the day (post-calibration) of measurement
- Use a correction factor (F) of 0.478 to compensate for the contribution of Sr-85 to the reading.
- Calculate the amount of Sr-82 in the sample using the following equation:
Sr-82 (μCi) = dose calibration reading (μCi) / [1 + (R) (F)]Example: dose calibrator reading (μCi) = 0.8; Sr85/Sr82 ratio (R) =1.48; correction factor (F) = 0.478.Sr-82 (μCi) = 0.8 / [1 + (1.48)(0.478)] = 0.47
- Determine if Sr-82 in the eluate exceeds an Alert or Expiration Limit by dividing the μCi of Sr-82 by the mCi of Rb-82 at End of Elution (see below for further instructions based on the Sr-82 level)
- Example: 0.47 μCi of Sr-82; 50 mCi of Rb-82 E.O.E.
0.47 μCi Sr-82 / 50 mCi Rb-82 = 0.0094 μCi/mCi Rb-82 (is above Alert Limit of 0.002; additional daily eluate testing must be performed)
- Determine if Sr-85 in the eluate exceeds an Alert or Expiration Limit by multiplying the result obtained in step 10 by (R) as calculated in step 7 (above).
- Example: 0.0094 x 1.48 = 0.014 μCi Sr-85/mCi Rb-82 (test result is below Alert and Expiration Limits)

CardioGen-82 Expiration
- Stop use of the CardioGen-82 generator once any one of the following Expiration Limits is reached.
- A total elution volume of 17 L has passed through the generator column, or
- 42 days post calibration date, or
- An eluate Sr-82 level of 0.01 μCi /mCi Rb-82, or
- An eluate Sr-85 level of 0.1 μCi /mCi Rb-82.
Radiation Dosimetry
- The estimated absorbed radiation doses for Rb-82, Sr-82, and Sr-85 from an intravenous injection rubidium Rb- 82 chloride are shown in Table 3.

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rubidium Rb 82 in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rubidium Rb 82 in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- safety and effectiveness not established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rubidium Rb 82 in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rubidium Rb 82 in pediatric patients.
Contraindications
- None
Warnings
|
WARNING: UNINTENDED STRONTIUM-82 (SR-82) AND STRONTIUM-85 (SR-85) RADIATION EXPOSURE:
See full prescribing information for complete Boxed Warning.
UNINTENDED STRONTIUM-82 (SR-82) AND STRONTIUM-85 (SR-85) RADIATION EXPOSURE:
Perform generator eluate tests: Record each generator eluate volume, including waste and test volumes, and keep a record of the cumulative eluate volume. Determine Rb-82, Sr-82, Sr-85 in the generator eluate: ●Once a day, prior to any drug administrations, and ●At additional daily tests after detection of an Alert Limit. Alert Limits are: ○14 L for the generator’s cumulative eluate volume, or ○An eluate Sr-82 level of 0.002 μCi/ mCi Rb-82, or ○An eluate Sr-85 level of 0.02 Sr-85 μCi/ mCi Rb-82. ○Perform the additional daily tests at time points determined by the day’s elution volume; tests are performed every 750 mL [see Dosage and Administration (2.5)]. Stop use of a generator at an Expiration Limit of: ○17 L for the generator’s cumulative eluate volume, or ○42 days post generator calibration date, or ○An eluate Sr-82 level of 0.01 μCi /mCi Rb-82, or ○An eluate Sr-85 level of 0.1 μCi /mCi Rb-82 |
Unintended Sr-82 and Sr-85 Exposure
- Unintended radiation exposure occurs when the Sr-82 and Sr-85 levels in rubidium Rb 82 chloride injections exceed the specified generator eluate limits. Unintended exposure to strontium radiation has occurred in some patients who received rubidium Rb 82 injections at clinical sites where generator eluate testing appeared insufficient. The physical half lives of Sr-82 and Sr-85 are 25 days and 65 days, respectively, in contrast to Rb-82 which has a physical half-life of 75 seconds. Unintended exposure to strontium radiation contributes to a patient’s overall cumulative radiation dose.
- To minimize the risk of unintended radiation exposure, strict adherence to a daily eluate testing protocol is required. Stop using the rubidium generator when the expiration limits are reached.
Risks Associated with Pharmacologic Stress
- Pharmacologic induction of cardiovascular stress may be associated with serious adverse reactions such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction, and cerebrovascular events. Perform pharmacologic stress testing in accordance with the pharmacologic stress agent’s prescribing information and only in the setting where cardiac resuscitation equipment and trained staff are readily available.
Volume Overload
- Patients with congestive heart failure or the elderly may experience a transitory increase in circulatory volume load. Observe these patients during infusion and for several hours following rubidium chloride injection administration to detect delayed hemodynamic disturbances.
Cumulative Radiation Exposure: Long-Term Risk of Cancer
- Rubidium Rb 82 chloride injection, similar to other radiopharmaceuticals, contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Use the lowest dose of rubidium Rb 82 chloride injection necessary for imaging and ensure safe handling to protect the patient and health care worker. Encourage patients to void as soon as a study is completed and as often as possible thereafter for at least one hour.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Rubidium Rb 82 in the drug label.
Postmarketing Experience
- The following serious adverse reactions have been identified during postapproval use of CardioGen-82. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Unintended radiation exposure has occurred in some patients who received rubidium Rb 82 chloride injections at clinical sites where generator eluate testing appeared insufficient
Drug Interactions
- Specific drug-drug interactions have not been studied.
Use in Specific Populations
Pregnancy
- Animal reproductive studies have not been conducted with rubidium Rb 82 chloride injection. It is also not known whether rubidium Rb 82 chloride injection can cause fetal harm when administered to a pregnant woman; however, all radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. If considering rubidium Rb 82 chloride injection administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from rubidium Rb-82 and the gestational timing of exposure. Administer rubidium Rb-82 to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rubidium Rb 82 in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rubidium Rb 82 during labor and delivery.
Nursing Mothers
- It is not known whether rubidium Rb 82 chloride injection is excreted in human milk. Due to the short half-life of rubidium Rb-82 (75 seconds) it is unlikely that the drug would be excreted in human milk during lactation. However, because many drugs are excreted in human milk, caution should be exercised when rubidium Rb-82 chloride injection is administered to nursing women. Do not resume breastfeeding until one hour after the last infusion.
Pediatric Use
- Rubidium Rb 82 chloride injection safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- In elderly patients with a clinically important decrease in cardiac function, lengthen the delay between infusion and image acquisition. Observe for the possibility of fluid overload
Gender
There is no FDA guidance on the use of Rubidium Rb 82 with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rubidium Rb 82 with respect to specific racial populations.
Renal Impairment
Reductions in renal function are not anticipated to alter clearance of rubidium Rb 82 chloride injection because Rb-82 decays to stable Kr-82 with a half-life of 75 seconds and Kr-82 is exhaled through the lungs.
Hepatic Impairment
Reductions in hepatic function are not anticipated to alter clearance of rubidium Rb 82 chloride injection.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rubidium Rb 82 in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rubidium Rb 82 in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Rubidium Rb 82 in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Rubidium Rb 82 in the drug label.
Overdosage
There is limited information regarding Overdose of Rubidium Rb 82 in the drug label.
Pharmacology
Template:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox E numberTemplate:Chembox OtherCationsTemplate:Chembox Supplement| Template:Chembox header2 | Rubidium Rb 82 | |
|---|---|
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| ClRb | |
| Molar mass | 117.371 g mol−1 |
| Related compounds | |
| Template:Chembox header2 | Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Mechanism of Action
- Rb-82 is analogous to potassium ion (K+) in its biochemical behavior and is rapidly extracted by the myocardium proportional to the blood flow. Rb+ participates in the sodium-potassium (Na+/K+) ion exchange pumps that are present in cell membranes. The intracellular uptake of Rb-82 requires maintenance of ionic gradient across cell membranes. Rb-82 radioactivity is increased in viable myocardium reflecting intracellular retention, while the tracer is cleared rapidly from necrotic or infarcted tissue.
Structure
Chemical Characteristics
- CardioGen-82 contains accelerator-produced Sr-82 adsorbed on stannic oxide in a lead-shielded column and provides a means for obtaining sterile nonpyrogenic solutions of rubidium Rb 82 chloride injection. The chemical form of Rb-82 is 82RbCl.
- The amount (millicuries) of Rb-82 obtained in each elution will depend on the potency of the generator.
- When eluted at a rate of 50 mL/minute, each generator eluate at the end of elution should not contain more than 0.02 microcurie of Sr-82 and not more than 0.2 microcurie of Sr-85 per millicurie of rubidium Rb 82 chloride injection, and not more than 1 microgram of tin per mL of eluate.
Physical Characteristics
- Rb-82 decays by positron emission and associated gamma emission with a physical half-life of 75 seconds.4 Table 4 shows the annihilation photons released following positron emission which are useful for detection and imaging studies.
- The decay modes of Rb-82 are: 95.5% by positron emission, resulting in the production of annihilation radiation, i.e., two 511 keV gamma rays; and 4.5% by electron capture, resulting in the emission of “prompt” gamma rays of predominantly 776.5 keV. Both decay modes lead directly to the formation of stable Kr-82.4
Pharmacodynamics
- In human studies, myocardial activity was noted within the first minute after peripheral intravenous injection of Rb-82. When areas of infarction or ischemia are present in the myocardium, they are visualized within 2-7 minutes after injection as photon-deficient, or “cold”, areas on the myocardial scan.
In patients with reduced cardiac function, transit of the injected dose from the peripheral infusion site to the myocardium may be delayed.
- Blood flow brings Rb-82 to all areas of the body during the first pass of circulation. Accordingly, visible uptake is also observed in other highly vascularized organs, such as the kidneys, liver, spleen and lungs.
Pharmacokinetics
- With a physical half-life of 75 seconds, Rb-82 is very rapidly converted by radioactive decay into a trace amount of stable Kr-82 gas, which is passively expired by the lungs. Renal and hepatic excretion is not anticipated to play an essential role in Rb-82 elimination, although some of the Rb-82 dose may be excreted in the urine prior to radioactive decay.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No long-term studies have been performed to evaluate carcinogenic potential, mutagenicity potential, or to determine whether rubidium Rb 82 chloride injection may affect fertility in males or females.
Clinical Studies
- In a descriptive, prospective, blinded image interpretation study6 of adult patients with known or suspected coronary artery disease, myocardial perfusion deficits in stress and rest PET images obtained with ammonia N 13 (n = 111) or rubidium Rb-82 chloride (n = 82) were compared to changes in stenosis flow reserve (SFR) as determined by coronary angiography. PET perfusion defects at rest and stress for seven cardiac regions (anterior, apical, anteroseptal, posteroseptal, anterolateral, posterolateral, and inferior walls) were graded on a scale of 0 (normal) to 5 (severe). Values for stenosis flow reserve, defined as flow at maximum coronary vasodilatation relative to rest flow, ranged from 0 (total occlusion) to 5 (normal). With increasing impairment of flow reserve, the subjective PET defect severity increased. A PET defect score of 2 or higher was positively correlated with flow reserve impairment (SFR<3).
- A systematic review of published literature was conducted using pre-defined inclusion/exclusion criteria which resulted in identification of 10 studies evaluating the use of Rb-82 PET myocardial perfusion imaging (MPI) for the identification of coronary artery disease as defined by catheter-based angiography. In these studies, the patient was the unit of analysis and 50% stenosis was the threshold for clinically significant coronary artery disease (CAD). Of these 10 studies, 9 studies were included in a meta-analysis for sensitivity (excluding one study with 100% sensitivity) and 7 studies were included in a meta-analysis of specificity (excluding 3 studies with 100% specificity). A random effects model yielded overall estimates of sensitivity and specificity of 92% (95% CI: 89% to 95%) and 81% (95% CI: 76% to 86%), respectively. The use of meta-analysis in establishing performance characteristics is limited, particularly by the possibility of publication bias (positive results being more likely to be published than negative results) which is difficult to detect especially when based on a limited number of small studies.
How Supplied
- CardioGen-82®(rubidium Rb 82 generator) consists of Sr-82 adsorbed on a hydrous stannic oxide column with an activity of 90-150 millicuries Sr-82 at calibration time. A lead shield surrounded by a labeled plastic container encases the generator. The container label provides complete assay data for each generator. Directions for determining the activity of Rb-82 eluted from the generator are described above [see Dosage and Administration (2.5)]. Use CardioGen-82 (rubidium Rb 82 Generator) only with an appropriate, properly calibrated infusion system labeled for use with the generator.
- Receipt, transfer, handling, possession or use of this product is subject to the radioactive material regulations and licensing requirements of the U.S. Nuclear Regulatory Commission, Agreement States or Licensing States as appropriate.
Disposal
- Licensee personnel should monitor the amount of radioactivity present within the generator prior to its disposal. Do not dispose of the generator in regular refuse systems. Store and/or dispose of the generator in accordance with the conditions of NRC radioactive materials license pursuant to 10 CFR, Part 20, or equivalent conditions pursuant to Agreement State Regulation. For questions about the disposal of the CardioGen-82 generator, contact Bracco Diagnostics Inc. at 1-800-447-6883, option 3.
Storage
- Store the generator at 20-25°C (68-77°F)
Images
Drug Images
{{#ask: Page Name::Rubidium Rb 82 |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
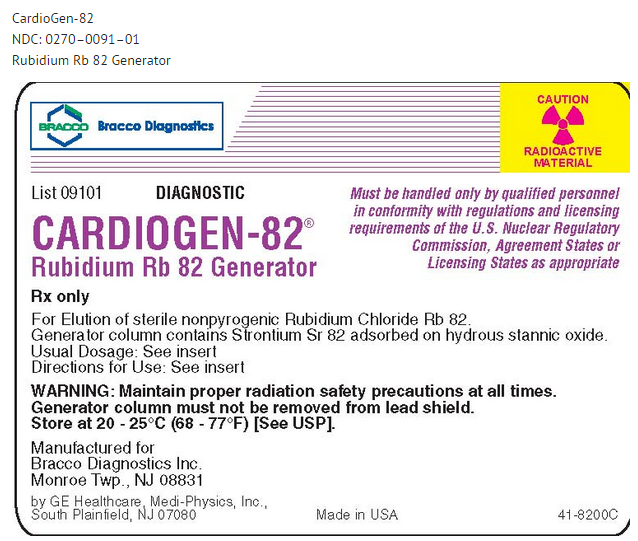
This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine.
Ingredients and Appearance
{{#ask: Label Page::Rubidium Rb 82 |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Women of Childbearing Potential
- Patients should be advised to inform their physician or healthcare provider if they are pregnant or breast feeding.
Post-study Breastfeeding Avoidance
- Instruct nursing patients to substitute stored breast milk or infant formula for breast milk for one hour after administration of rubidium Rb 82 chloride injection.
Post-study Voiding
- Instruct patients to void after completion of each image acquisition session and as often as possible for one hour after completion of the PET scan.
Precautions with Alcohol
- Alcohol-Rubidium Rb 82 interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CARDIOGEN-82®[1]
Look-Alike Drug Names
There is limited information regarding look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.