Rosiglitazone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2], Rabin Bista, M.B.B.S. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Rosiglitazone is a thiazolidinedione that is FDA approved for the treatment of as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Common adverse reactions include edema, weight gain, headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Glycemic Control in Adults with Type 2 Diabetes Mellitus
- Dosing information
- Starting dosage: 4 mg PO qd or bid
- For patients who respond inadequately following 8 to 12 weeks of treatment, as determined by reduction in fasting plasma glucose (FPG), the dose may be increased to 8 mg daily. Increases in the dose of rosiglitazone should be accompanied by careful monitoring for adverse events related to fluid retention. Rosiglitazone may be taken with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of rosiglitazone in adult patients.
Non–Guideline-Supported Use
Coronary Stent Stenosis
- Dosing information
- 4 mg orally daily[1]
Insulin Resistance
- Dosing information
- 4 mg daily [2]
Metabolic Syndrome
- Dosing information
- 4 mg daily for 8 weeks[3]
Non-Alcoholic Fatty Liver
- Dosing information
- 4 mg daily for 1 month, then 8 mg daily thereafter[4]
Polycystic Ovary Syndrome
- Dosing information
- 4 mg twice daily[5]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Glycemic Control in Adults with Type 2 Diabetes Mellitus
- Dosing information
- Starting dosage: 4 mg PO qd or bid
- For patients who respond inadequately following 8 to 12 weeks of treatment, as determined by reduction in fasting plasma glucose (FPG), the dose may be increased to 8 mg daily. Increases in the dose of rosiglitazone should be accompanied by careful monitoring for adverse events related to fluid retention. Rosiglitazone may be taken with or without food.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of rosiglitazone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of rosiglitazone in pediatric patients.
Contraindications
- Initiation of rosiglitazone in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated.
- Use in patients with a history of a hypersensitivity reaction to rosiglitazone or any of the product’s ingredients.
Warnings
Cardiac Failure
- Rosiglitazone, like other thiazolidinediones, alone or in combination with other anti diabetic agents, can cause fluid retention, which may exacerbate or lead to heart failure. Patients should be observed for signs and symptoms of heart failure. If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of rosiglitazone must be considered.
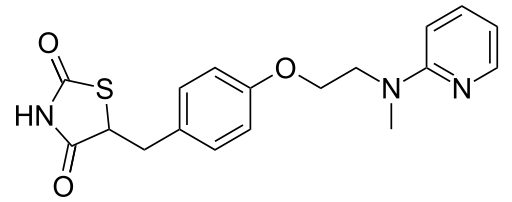
Patients with congestive heart failure (CHF) NYHA Class I and II treated with rosiglitazone have an increased risk of cardiovascular events. A 52-week, double-blind, placebo-controlled, echocardiographic trial was conducted in 224 patients with type 2 diabetes mellitus and NYHA Class I or II CHF (ejection fraction ≤45%) on background anti diabetic and CHF therapy. An independent committee conducted a blinded evaluation of fluid-related events (including congestive heart failure) and cardiovascular hospitalizations according to predefined criteria (adjudication). Separate from the adjudication, other cardiovascular adverse events were reported by investigators. Although no treatment difference in change from baseline of ejection fractions was observed, more cardiovascular adverse events were observed following treatment with rosiglitazone compared with placebo during the 52-week trial. (See Table 1.)
- In a long-term, cardiovascular outcome trial (RECORD) in patients with type 2 diabetes , the incidence of heart failure was higher in patients treated with rosiglitazone [2.7% (61/2,220) compared with active control 1.3% (29/2,227), HR 2.10 (95% CI: 1.35, 3.27)].
Initiation of rosiglitazone in patients with established NYHA Class III or IV heart failure is contraindicated. Rosiglitazone is not recommended in patients with symptomatic heart failure.
- Patients experiencing acute coronary syndromes have not been studied in controlled clinical trials. In view of the potential for development of heart failure in patients having an acute coronary event, initiation of rosiglitazone is not recommended for patients experiencing an acute coronary event, and discontinuation of rosiglitazone during this acute phase should be considered.
- Patients with NYHA Class III and IV cardiac status (with or without CHF) have not been studied in controlled clinical trials. Rosiglitazone is not recommended in patients with NYHA Class III and IV cardiac status.
Congestive Heart Failure During Coadministration of Rosiglitazone with Insulin: In trials in which rosiglitazone was added to insulin, rosiglitazone increased the risk of congestive heart failure. Coadministration of rosiglitazone and insulin is not recommended.
In 7 controlled, randomized, double-blind trials which had durations from 16 to 26 weeks and which were included in a meta-analysis , patients with type 2 diabetes mellitus were randomized to coadministration of rosiglitazone and insulin (N = 1,018) or insulin (N = 815). In these 7 trials, rosiglitazone was added to insulin. These trials included patients with long-standing diabetes (median duration of 12 years) and a high prevalence of pre-existing medical conditions, including peripheral neuropathy, retinopathy, ischemic heart disease, vascular disease, and congestive heart failure. The total number of patients with emergent congestive heart failure was 23 (2.3%) and 8 (1.0%) in the group receiving rosiglitazone plus insulin and the insulin group, respectively.
Heart Failure in Observational Studies of Elderly Diabetic Patients Comparing Rosiglitazone to Pioglitazone: Three observational studies in elderly diabetic patients (age 65 years and older) found that rosiglitazone statistically significantly increased the risk of hospitalized heart failure compared to use of pioglitazone. One other observational study in patients with a mean age of 54 years, which also included an analysis in a sub population of patients >65 years of age, found no statistically significant increase in emergency department visits or hospitalization for heart failure in patients treated with rosiglitazone compared to pioglitazone in the older subgroup.
Major Adverse Cardiovascular Events
- Data from long-term, prospective, randomized, controlled clinical trials of rosiglitazone versus metformin or sulfonylureas, particularly a cardiovascular outcome trial (RECORD), observed no difference in overall mortality or in major adverse cardiovascular events (MACE) and its components. A meta-analysis of mostly short-term trials suggested an increased risk for myocardial infarction with rosiglitazone compared with placebo.
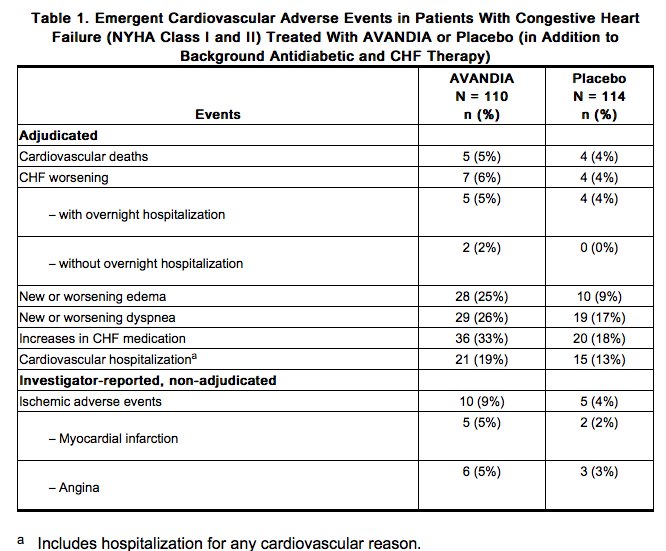
Cardiovascular Events in Large, Long-term, Prospective, Randomized, Controlled Trials of Rosiglitazone: RECORD, a prospectively designed cardiovascular outcome trial (mean follow-up 5.5 years; 4,447 patients), compared the addition of rosiglitazone to metformin or a sulfonylurea (N = 2,220) with a control group of metformin plus sulfonylurea(N = 2,227) in patients with type 2 diabetes . Non-inferiority was demonstrated for the primary endpoint, cardiovascular hospitalization or cardiovascular death, for rosiglitazone compared with control [HR 0.99 (95% CI: 0.85, 1.16)] demonstrating no overall increased risk in cardiovascular morbidity or mortality. The hazard ratios for total mortality and MACE were consistent with the primary endpoint and the 95% CI similarly excluded a 20% increase in risk for rosiglitazone. The hazard ratios for the components of MACE were 0.72 (95% CI: 0.49, 1.06) for stroke, 1.14 (95% CI: 0.80, 1.63) for myocardial infarction, and 0.84 (95% CI: 0.59, 1.18) for cardiovascular death.
- The results of RECORD are consistent with the findings of 2 earlier long-term, prospective, randomized, controlled clinical trials (each trial >3 years’ duration; total of 9,620 patients) (see Figure 1). In patients with impaired glucose tolerance (DREAM trial), although the incidence of cardiovascular events was higher among subjects who were randomized to rosiglitazone in combination with ramipril than among subjects randomized to ramipril alone, no statistically significant differences were observed for MACE and its components between rosiglitazone and placebo. In type 2 diabetes patients who were initiating oral agent monotherapy (ADOPT trial), no statistically significant differences were observed for MACE and its components between rosiglitazone and metformin or a sulfonylurea.
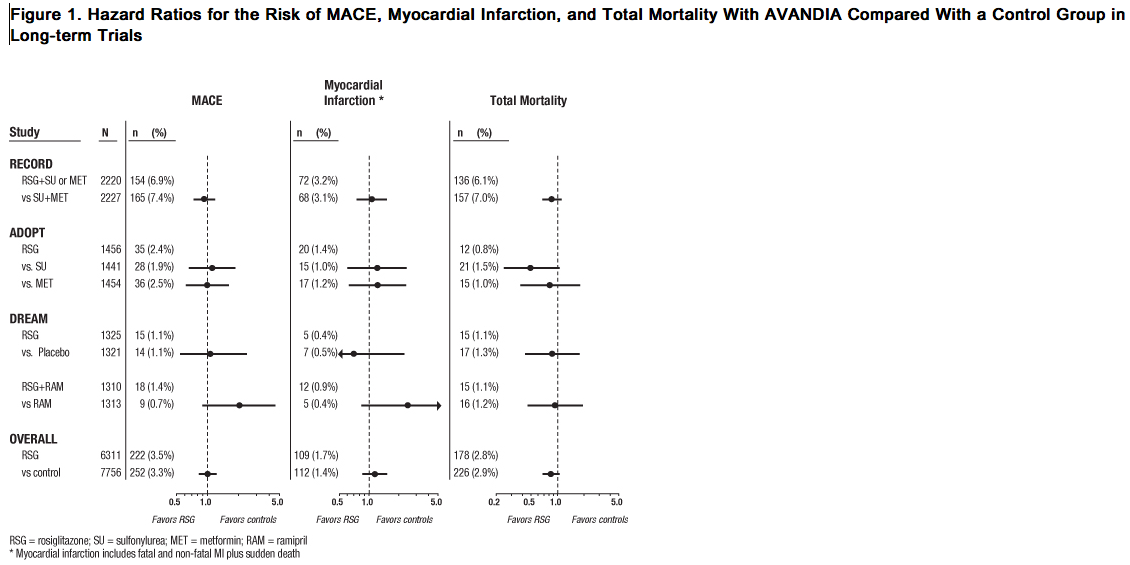
- Cardiovascular Events in a Group of 52 Clinical Trials: In a meta-analysis of 52 double-blind, randomized, controlled clinical trials designed to assess glucose-lowering efficacy in type 2 diabetes (mean duration 6 months), a statistically significant increased risk of myocardial infarction with rosiglitazone versus pooled comparators was observed [0.4% versus 0.3%; OR 1.8, (95% CI: 1.03, 3.25)]. A statistically non-significant increased risk of MACE was observed with rosiglitazone versus pooled comparators (OR 1.44, 95% CI: 0.95, 2.20). In the placebo-controlled trials, a statistically significant increased risk of myocardial infarction [0.4% versus 0.2%, OR 2.23 (95% CI: 1.14, 4.64)] and statistically non-significant increased risk of MACE [0.7% versus 0.5%, OR 1.53 (95% CI: 0.94, 2.54)] with rosiglitazone were observed. In the active-controlled trials, there was no increased risk of myocardial infarction or MACE.
Mortality in Observational Studies of rosiglitazone compared to pioglitazone: Three observational studies in elderly diabetic patients (age 65 years and older) found that rosiglitazone statistically significantly increased the risk of all-cause mortality compared to use of pioglitazone. One observational study in patients with a mean age of 54 years found no difference in all-cause mortality between patients treated with rosiglitazone compared to pioglitazone and reported similar results in the sub population of patients >65 years of age. One additional small, prospective, observational study found no statistically significant differences for CV mortality and all-cause mortality in patients treated with rosiglitazone compared to pioglitazone.
Edema
- Rosiglitazone should be used with caution in patients with edema. In a clinical trial in healthy volunteers who received 8 mg of rosiglitazone once daily for 8 weeks, there was a statistically significant increase in median plasma volume compared with placebo.
- Since thiazolidinediones, including rosiglitazone, can cause fluid retention, which can exacerbate or lead to congestive heart failure, rosiglitazone should be used with caution in patients at risk for heart failure. Patients should be monitored for signs and symptoms of heart failure.
- In controlled clinical trials of patients with type 2 diabetes, mild to moderate edema was reported in patients treated with rosiglitazone, and may be dose related. Patients with ongoing edema were more likely to have adverse events associated with edema if started on combination therapy with insulin and rosiglitazone.
Weight Gain
- Dose-related weight gain was seen with rosiglitazone alone and in combination with other hypoglycemic agents (Table 2). The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation.
- In post marketing experience, there have been reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive edema and congestive heart failure.
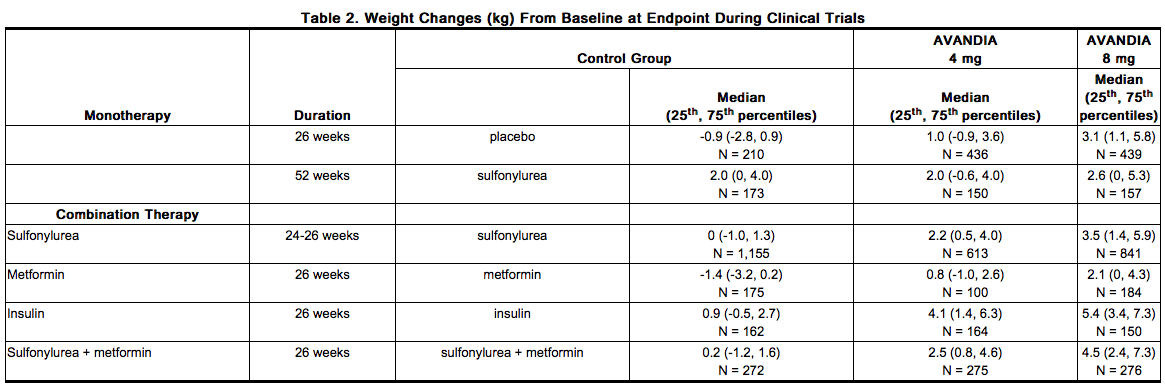
- In a 4- to 6-year, monotherapy, comparative trial (ADOPT) in patients recently diagnosed with type 2 diabetes not previously treated with anti diabetic medication , the median weight change (25th, 75th percentiles) from baseline at 4 years was 3.5 kg (0.0, 8.1) for rosiglitazone, 2.0 kg (-1.0, 4.8) for glyburide, and -2.4 kg (-5.4, 0.5) for metformin.
- In a 24-week trial in pediatric patients aged 10 to 17 years treated with rosiglitazone 4 to 8 mg daily, a median weight gain of 2.8 kg (25th, 75th percentiles: 0.0, 5.8) was reported.
Hepatic Effects
- Liver enzymes should be measured prior to the initiation of therapy with rosiglitazone in all patients and periodically thereafter per the clinical judgment of the healthcare professional. therapy with rosiglitazone should not be initiated in patients with increased baseline liver enzyme levels (ALT >2.5X upper limit of normal). Patients with mildly elevated liver enzymes (ALT levels ≤2.5X upper limit of normal) at baseline or during therapy with rosiglitazone should be evaluated to determine the cause of the liver enzyme elevation. Initiation of, or continuation of, therapy with rosiglitazone in patients with mild liver enzyme elevations should proceed with caution and include close clinical follow-up, including liver enzyme monitoring, to determine if the liver enzyme elevations resolve or worsen. If at any time ALT levels increase to >3X the upper limit of normal in patients on therapy with rosiglitazone, liver enzyme levels should be rechecked as soon as possible. If ALT levels remain >3X the upper limit of normal, therapy with rosiglitazone should be discontinued.
- If any patient develops symptoms suggesting hepatic dysfunction, which may include unexplained nausea, vomiting, abdominal pain, fatigue, anorexia and/or dark urine, liver enzymes should be checked. The decision whether to continue the patient on therapy with rosiglitazone should be guided by clinical judgment pending laboratory evaluations. If jaundice is observed, drug therapy should be discontinued.
Macular Edema
- Macular edema has been reported in post marketing experience in some diabetic patients who were taking rosiglitazone or another thiazolidinedione. Some patients presented with blurred vision or decreased visual acuity, but some patients appear to have been diagnosed on routine ophthalmologic examination. Most patients had peripheral edema at the time macular edema was diagnosed. Some patients had improvement in their macular edema after discontinuation of their thiazolidinedione. Patients with diabetes should have regular eye exams by an ophthalmologist, per the Standards of Care of the American Diabetes Association. Additionally, any diabetic who reports any kind of visual symptom should be promptly referred to an ophthalmologist, regardless of the patient’s underlying medications or other physical findings.
Fractures
- Long-term trials (ADOPT and RECORD) show an increased incidence of bone fracture in patients, particularly female patients, taking rosiglitazone . This increased incidence was noted after the first year of treatment and persisted during the course of the trial. The majority of the fractures in the women who received rosiglitazone occurred in the upper arm, hand, and foot. These sites of fracture are different from those usually associated with post menopausal osteoporosis (e.g., hip or spine). Other trials suggest that this risk may also apply to men, although the risk of fracture among women appears higher than that among men. The risk of fracture should be considered in the care of patients treated with rosiglitazone, and attention given to assessing and maintaining bone health according to current standards of care.
Hematologic Effects
- Decreases in mean hemoglobin and hematocrit occurred in a dose-related fashion in adult patients treated with rosiglitazone . The observed changes may be related to the increased plasma volume observed with treatment with rosiglitazone.
Diabetes and Blood Glucose Control
- Patients receiving rosiglitazone in combination with other hypoglycemic agents may be at risk for hypoglycemia, and a reduction in the dose of the concomitant agent may be necessary.
- Periodic fasting blood glucose and HbA1c measurements should be performed to monitor therapeutic response.
Ovulation
- Therapy with rosiglitazone, like other thiazolidinediones, may result in ovulation in some pre menopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking rosiglitazone. Thus, adequate contraception in premenopausal women should be recommended. This possible effect has not been specifically investigated in clinical trials; therefore, the frequency of this occurrence is not known.
- Although hormonal imbalance has been seen in preclinical studies , the clinical significance of this finding is not known. If unexpected menstrual dysfunction occurs, the benefits of continued therapy with rosiglitazone should be reviewed.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult: In clinical trials, approximately 9,900 patients with type 2 diabetes have been treated with rosiglitazone.
- Short-term Trials of Rosiglitazone as Monotherapy and in Combination With Other Hypoglycemic Agents: The incidence and types of adverse events reported in short-term clinical trials of rosiglitazone as monotherapy are shown in Table 3.
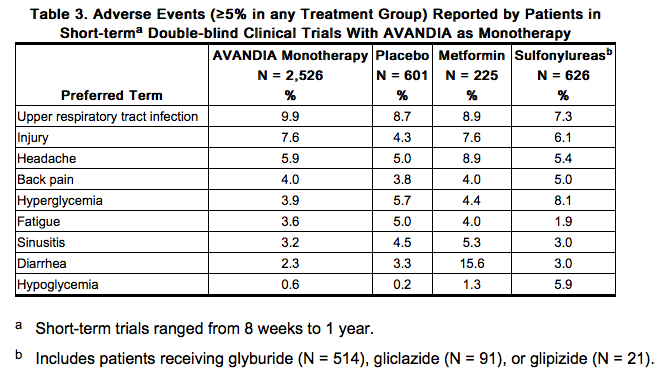
- Overall, the types of adverse reactions without regard to causality reported when rosiglitazone was used in combination with a sulfonylurea or metformin were similar to those during monotherapy with rosiglitazone.
- Events of anemia and edema tended to be reported more frequently at higher doses, and were generally mild to moderate in severity and usually did not require discontinuation of treatment with rosiglitazone.
- In double-blind trials, anemia was reported in 1.9% of patients receiving rosiglitazone as monotherapy compared with 0.7% on placebo, 0.6% on sulfonylureas, and 2.2% on metformin. Reports of anemia were greater in patients treated with a combination of rosiglitazone and metformin (7.1%) and with a combination of rosiglitazone and a sulfonylurea plus metformin (6.7%) compared with monotherapy with rosiglitazone or in combination with a sulfonylurea (2.3%). Lower pre-treatment hemoglobin/hematocrit levels in patients enrolled in the metformin combination clinical trials may have contributed to the higher reporting rate of anemia in these trials .
- In clinical trials, edema was reported in 4.8% of patients receiving rosiglitazone as monotherapy compared with 1.3% on placebo, 1.0% on sulfonylureas, and 2.2% on metformin. The reporting rate of edema was higher for rosiglitazone 8 mg in sulfonylurea combinations (12.4%) compared with other combinations, with the exception of insulin. Edema was reported in 14.7% of patients receiving rosiglitazone in the insulin combination trials compared with 5.4% on insulin alone. Reports of new onset or exacerbation of congestive heart failure occurred at rates of 1% for insulin alone, and 2% (4 mg) and 3% (8 mg) for insulin in combination with rosiglitazone .
- In controlled combination therapy trials with sulfonylureas, mild to moderate hypoglycemic symptoms, which appear to be dose related, were reported. Few patients were withdrawn for hypoglycemia (<1%) and few episodes of hypoglycemia were considered to be severe (<1%). Hypoglycemia was the most frequently reported adverse event in the fixed-dose insulin combination trials, although few patients withdrew for hypoglycemia (4 of 408 for rosiglitazone plus insulin and 1 of 203 for insulin alone). Rates of hypoglycemia, confirmed by capillary blood glucose concentration ≤50 mg/dL, were 6% for insulin alone and 12% (4 mg) and 14% (8 mg) for insulin in combination with rosiglitazone.
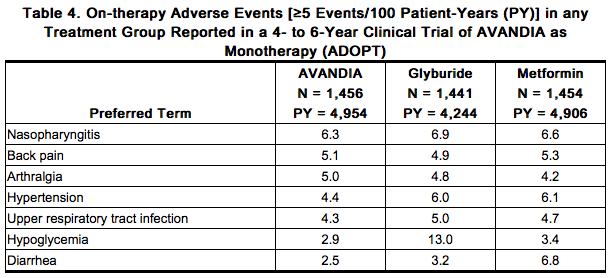
- Long-term trial of rosiglitazone as Monotherapy: A 4- to 6-year trial (ADOPT) compared the use of rosiglitazone (n = 1,456), glyburide (n = 1,441), and metformin (n = 1,454) as monotherapy in patients recently diagnosed with type 2 diabetes who were not previously treated with antidiabetic medication. Table 4 presents adverse reactions without regard to causality; rates are expressed per 100 patient-years (PY) exposure to account for the differences in exposure to trial medication across the 3 treatment groups.
- In ADOPT, fractures were reported in a greater number of women treated with rosiglitazone (9.3%, 2.7/100 patient-years) compared with glyburide (3.5%, 1.3/100 patient-years) or metformin (5.1%, 1.5/100 patient-years). The majority of the fractures in the women who received rosiglitazone were reported in the upper arm, hand, and foot. The observed incidence of fractures for male patients was similar among the 3 treatment groups.
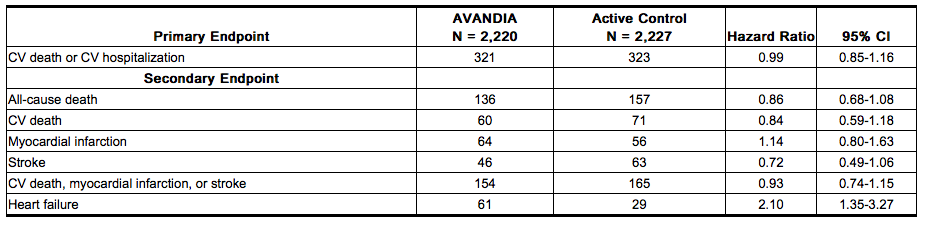
Long-term Trial of Rosiglitazone as Combination Therapy (RECORD): RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes) was a multicenter, randomized, open-label, non-inferiority trial in subjects with type 2 diabetes inadequately controlled on maximum doses of metformin or sulfonylurea (glyburide, gliclazide, or glimepiride) to compare the time to reach the combined cardiovascular endpoint of cardiovascular death or cardiovascular hospitalization between patients randomized to the addition of rosiglitazone versus metformin or sulfonylurea. The trial included patients who have failed metformin or sulfonylurea monotherapy; those who failed metformin (n = 2,222) were randomized to receive either rosiglitazone as add-on therapy (n = 1,117) or add-on sulfonylurea (n = 1,105), and those who failed sulfonylurea (n = 2,225) were randomized to receive either rosiglitazone as add-on therapy (n = 1,103) or add-on metformin (n = 1,122). Patients were treated to target HbA1c ≤7% throughout the trial.
- The mean age of patients in this trial was 58 years, 52% were male, and the mean duration of follow-up was 5.5 years. Rosiglitazone demonstrated non-inferiority to active control for the primary endpoint of cardiovascular hospitalization or cardiovascular death (HR 0.99, 95% CI: 0.85-1.16). There were no significant differences between groups for secondary endpoints with the exception of congestive heart failure (see Table 5). The incidence of congestive heart failure was significantly greater among patients randomized to rosiglitazone.
- There was an increased incidence of bone fracture for subjects randomized to rosiglitazone in addition to metformin or sulfonylurea compared with those randomized to metformin plus sulfonylurea (8.3% versus 5.3%) . The majority of fractures were reported in the upper limbs and distal lower limbs. The risk of fracture appeared to be higher in females relative to control (11.5% versus 6.3%), than in males relative to control (5.3% versus 4.3%). Additional data are necessary to determine whether there is an increased risk of fracture in males after a longer period of follow-up.
Pediatric: Rosiglitazone has been evaluated for safety in a single, active-controlled trial of pediatric patients with type 2 diabetes in which 99 were treated with rosiglitazone and 101 were treated with metformin. The most common adverse reactions (>10%) without regard to causality for either rosiglitazone or metformin were headache (17% versus 14%), nausea (4% versus 11%), nasopharyngitis (3% versus 12%), and diarrhea (1% versus 13%). In this trial, one case of diabetic ketoacidosis was reported in the metformin group. In addition, there were 3 patients in the rosiglitazone group who had FPG of approximately 300 mg/dL, 2+ ketonuria, and an elevated anion gap.
Laboratory Abnormalities
Hematologic: Decreases in mean hemoglobin and hematocrit occurred in a dose-related fashion in adult patients treated with rosiglitazone (mean decreases in individual trials as much as 1.0 g/dL hemoglobin and as much as 3.3% hematocrit). The changes occurred primarily during the first 3 months following initiation of therapy with rosiglitazone or following a dose increase in rosiglitazone. The time course and magnitude of decreases were similar in patients treated with a combination of rosiglitazone and other hypoglycemic agents or monotherapy with rosiglitazone. Pre-treatment levels of hemoglobin and hematocrit were lower in patients in metformin combination trials and may have contributed to the higher reporting rate of anemia. In a single trial in pediatric patients, decreases in hemoglobin and hematocrit (mean decreases of 0.29 g/dL and 0.95%, respectively) were reported. Small decreases in hemoglobin and hematocrit have also been reported in pediatric patients treated with rosiglitazone. White blood cell counts also decreased slightly in adult patients treated with rosiglitazone. Decreases in hematologic parameters may be related to increased plasma volume observed with treatment with rosiglitazone. Lipids: Changes in serum lipids have been observed following treatment with rosiglitazone in adults. Small changes in serum lipid parameters were reported in children treated with rosiglitazone for 24 weeks. Serum Transaminase Levels: In pre-approval clinical trials in 4,598 patients treated with rosiglitazone (3,600 patient-years of exposure) and in a long-term 4- to 6-year trial in 1,456 patients treated with rosiglitazone (4,954 patient-years exposure), there was no evidence of drug-induced hepatotoxicity. In pre-approval controlled trials, 0.2% of patients treated with rosiglitazone had elevations in ALT >3X the upper limit of normal compared with 0.2% on placebo and 0.5% on active comparators. The ALT elevations in patients treated with rosiglitazone were reversible. Hyperbilirubinemia was found in 0.3% of patients treated with rosiglitazone compared with 0.9% treated with placebo and 1% in patients treated with active comparators. In pre-approval clinical trials, there were no cases of idiosyncratic drug reactions leading to hepatic failure.
- In the 4- to 6-year ADOPT trial, patients treated with Rosiglitazone (4,954 patient-years exposure), glyburide (4,244 patient-years exposure), or metformin (4,906 patient-years exposure), as monotherapy, had the same rate of ALT increase to >3X upper limit of normal (0.3 per 100 patient-years exposure).
In the RECORD trial, patients randomized to rosiglitazone in addition to metformin or sulfonylurea (10,849 patient-years exposure) and to metformin plus sulfonylurea (10,209 patient-years exposure) had a rate of ALT increase to ≥3X upper limit of normal of approximately 0.2 and 0.3 per 100 patient-years exposure, respectively.
Postmarketing Experience
- In addition to adverse reactions reported from clinical trials, the events described below have been identified during post-approval use of rosiglitazone. Because these events are reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or to always establish a causal relationship to drug exposure.
- In patients receiving thiazolidinedione therapy, serious adverse events with or without a fatal outcome, potentially related to volume expansion (e.g., congestive heart failure, pulmonary edema, and pleural effusions) have been reported .
- There are postmarketing reports with rosiglitazone of hepatitis, hepatic enzyme elevations to 3 or more times the upper limit of normal, and hepatic failure with and without fatal outcome, although causality has not been established.
- There are postmarketing reports with rosiglitazone of rash, pruritus, urticaria, angioedema, anaphylactic reaction, Stevens-Johnson syndrome , and new onset or worsening diabetic macular edema with decreased visual acuity.
Drug Interactions
CYP2C8 Inhibitors and Inducers
- An inhibitor of CYP2C8 (e.g., gemfibrozil) may increase the AUC of rosiglitazone and an inducer of CYP2C8 (e.g., rifampin) may decrease the AUC of rosiglitazone. Therefore, if an inhibitor or an inducer of CYP2C8 is started or stopped during treatment with rosiglitazone, changes in diabetes treatment may be needed based upon clinical response.
Use in Specific Populations
Pregnancy
- All pregnancies have a background risk of birth defects, loss, or other adverse outcome regardless of drug exposure. This background risk is increased in pregnancies complicated by hyperglycemia and may be decreased with good metabolic control. It is essential for patients with diabetes or history of gestational diabetes to maintain good metabolic control before conception and throughout pregnancy. Careful monitoring of glucose control is essential in such patients. Most experts recommend that insulin monotherapy be used during pregnancy to maintain blood glucose levels as close to normal as possible.
Human Data: Rosiglitazone has been reported to cross the human placenta and be detectable in fetal tissue. The clinical significance of these findings is unknown. There are no adequate and well-controlled trials in pregnant women. Rosiglitazone should be used during pregnancy only if the potential benefit justifies the potentialrisk to the fetus.
Animal Studies: There was no effect on implantation or the embryo with rosiglitazone treatment during early pregnancy in rats, but treatment during mid-late gestation was associated with fetal death and growth retardation in both rats and rabbits. Teratogenicity was not observed at doses up to 3 mg/kg in rats and 100 mg/kg in rabbits (approximately 20 and 75 times human AUC at the maximum recommended human daily dose, respectively). Rosiglitazone caused placental pathology in rats (3 mg/kg/day). Treatment of rats during gestation through lactation reduced litter size, neonatal viability, and postnatal growth, with growth retardation reversible after puberty. For effects on the placenta, embryo/fetus, and offspring, the no-effect dose was 0.2 mg/kg/day in rats and 15 mg/kg/day in rabbits. These no-effect levels are approximately 4 times human AUC at the maximum recommended human daily dose. Rosiglitazone reduced the number of uterine implantations and live offspring when juvenile female rats were treated at 40 mg/kg/day from 27 days of age through to sexual maturity (approximately 68 times human AUC at the maximum recommended daily dose). The no-effect level was 2 mg/kg/day (approximately 4 times human AUC at the maximum recommended daily dose). There was no effect on pre- or post-natal survival or growth.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rosiglitazone in women who are pregnant.
Labor and Delivery
The effect of rosiglitazone on labor and delivery in humans is not known.
Nursing Mothers
- Drug-related material was detected in milk from lactating rats. It is not known whether rosiglitazone is excreted in human milk. Because many drugs are excreted in human milk, a decision should be made whether to discontinue nursing or to discontinue rosiglitazone, taking into account the importance of the drug to the mother.
Pediatric Use
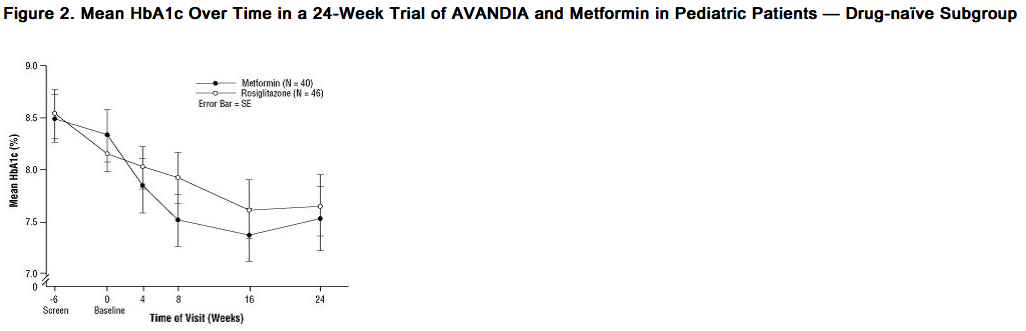
- After placebo run-in including diet counseling, children with type 2 diabetes mellitus, aged 10 to 17 years and with a baseline mean body mass index (BMI) of 33 kg/m2, were randomized to treatment with 2 mg twice daily of rosiglitazone (n = 99) or 500 mg twice daily of metformin (n = 101) in a 24-week, double-blind clinical trial. As expected, FPG decreased in patients naïve to diabetes medication (n = 104) and increased in patients withdrawn from prior medication (usually metformin) (n = 90) during the run-in period. After at least 8 weeks of treatment, 49% of patients treated with Rosiglitazone and 55% of metformin-treated patients had their dose doubled if FPG >126 mg/dL. For the overall intent-to-treat population, at Week 24, the mean change from baseline in HbA1c was -0.14% with rosiglitazone and -0.49% with metformin. There was an insufficient number of patients in this trial to establish statistically whether these observed mean treatment effects were similar or different. Treatment effects differed for patients naïve to therapy with antidiabetic drugs and for patients previously treated with antidiabetic therapy (Table 6).

- Treatment differences depended on baseline BMI or weight such that the effects of rosiglitazone and metformin appeared more closely comparable among heavier patients. The median weight gain was 2.8 kg with rosiglitazone and 0.2 kg with metformin. Fifty-four percent of patients treated with rosiglitazone and 32% of patients treated with metformin gained ≥2 kg, and 33% of patients treated with rosiglitazone and 7% of patients treated with metformin gained ≥5 kg on trial.
Adverse events observed in this trial are described in Adverse Reactions (6.1).
Geriatic Use
- Results of the population pharmacokinetic analysis showed that age does not significantly affect the pharmacokinetics of rosiglitazone. Therefore, no dosage adjustments are required for the elderly. In controlled clinical trials, no overall differences in safety and effectiveness between older (≥65 years) and younger (<65 years) patients were observed.
Gender
There is no FDA guidance on the use of Rosiglitazone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rosiglitazone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Rosiglitazone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Rosiglitazone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rosiglitazone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rosiglitazone in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
FDA Package Insert for Rosiglitazone contains no information regarding Drug Monitoring.
IV Compatibility
There is limited information about the IV compatibility.
Overdosage
- Limited data are available with regard to overdosage in humans. In clinical trials in volunteers, rosiglitazone has been administered at single oral doses of up to 20 mg and was well tolerated. In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s clinical status.
Pharmacology
Mechanism of Action
- Rosiglitazone, a member of the thiazolidinedione class of antidiabetic agents, improves glycemic control by improving insulin sensitivity. Rosiglitazone is a highly selective and potent agonist for the peroxisome proliferator-activated receptor-gamma (PPARγ). In humans, PPAR receptors are found in key target tissues for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors regulates the transcription of insulin-responsive genes involved in the control of glucose production, transport, and utilization. In addition, PPARγ-responsive genes also participate in the regulation of fatty acid metabolism.
- Insulin resistance is a common feature characterizing the pathogenesis of type 2 diabetes. The antidiabetic activity of rosiglitazone has been demonstrated in animal models of type 2 diabetes in which hyperglycemia and/or impaired glucose tolerance is a consequence of insulin resistance in target tissues. Rosiglitazone reduces blood glucose concentrations and reduces hyperinsulinemia in the ob/ob obese mouse, db/db diabetic mouse, and fa/fa fatty Zucker rat.
- In animal models, the antidiabetic activity of rosiglitazone was shown to be mediated by increased sensitivity to insulin’s action in the liver, muscle, and adipose tissues. Pharmacological studies in animal models indicate that rosiglitazone inhibits hepatic gluconeogenesis. The expression of the insulin-regulated glucose transporter GLUT-4 was increased in adipose tissue. Rosiglitazone did not induce hypoglycemia in animal models of type 2 diabetes and/or impaired glucose tolerance.
Structure
- Rosiglitazone (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. Rosiglitazone improves glycemic control while reducing circulating insulin levels.
- Rosiglitazone maleate is not chemically or functionally related to the sulfonylureas, the biguanides, or the alpha-glucosidase inhibitors.
Chemically, rosiglitazone maleate is (±)-5-[ [4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione, (Z)-2-butenedioate (1:1) with a molecular weight of 473.52 (357.44 free base). The molecule has a single chiral center and is present as a racemate. Due to rapid interconversion, the enantiomers are functionally indistinguishable. The structural formula of rosiglitazone maleate is:
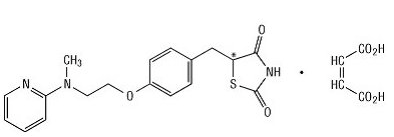
- The molecular formula is C18H19N3O3S•C4H4O4. Rosiglitazone maleate is a white to off-white solid with a melting point range of 122° to 123°C. The pKa values of rosiglitazone maleate are 6.8 and 6.1. It is readily soluble in ethanol and a buffered aqueous solution with pH of 2.3; solubility decreases with increasing pH in the physiological range.
- Each pentagonal film-coated TILTAB tablet contains rosiglitazone maleate equivalent to rosiglitazone, 2 mg, 4 mg, or 8 mg, for oral administration. Inactive ingredients are: hypromellose 2910, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol 3000, sodium starch glycolate, titanium dioxide, triacetin, and 1 or more of the following: synthetic red and yellow iron oxides and talc.
Pharmacodynamics
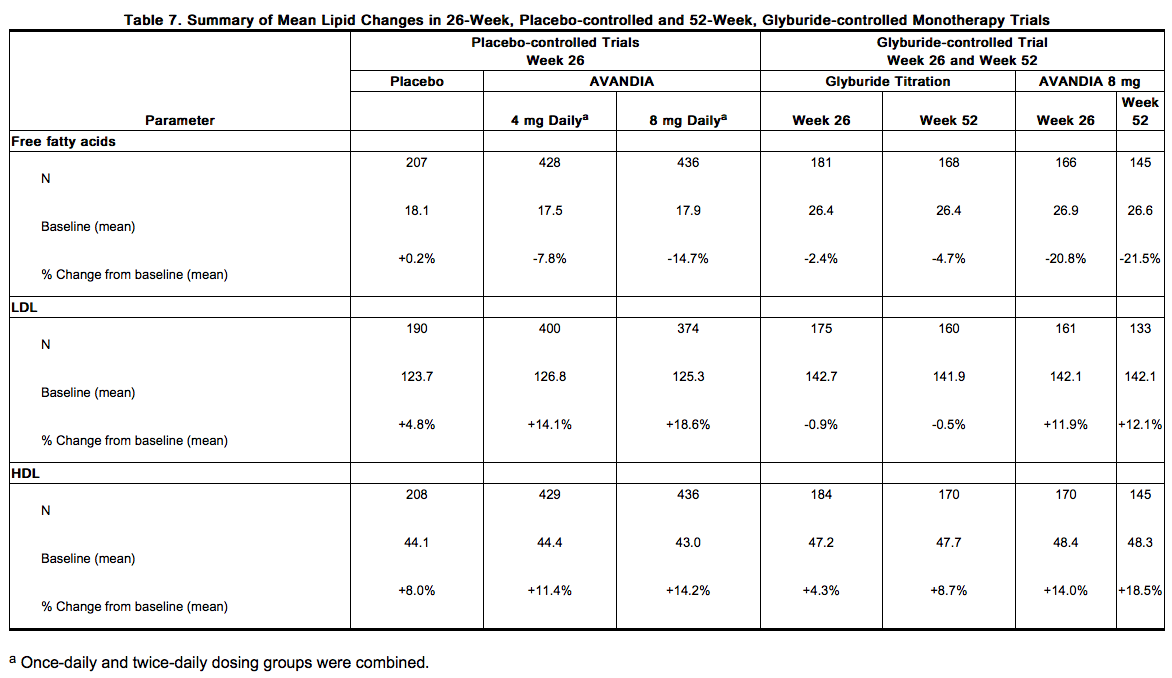
- Patients with lipid abnormalities were not excluded from clinical trials of rosiglitazone. In all 26-week controlled trials, across the recommended dose range, rosiglitazone as monotherapy was associated with increases in total cholesterol, LDL, and HDL and decreases in free fatty acids. These changes were statistically significantly different from placebo or glyburide controls (Table 7).
- Increases in LDL occurred primarily during the first 1 to 2 months of therapy with rosiglitazone and LDL levels remained elevated above baseline throughout the trials. In contrast, HDL continued to rise over time. As a result, the LDL/HDL ratio peaked after 2 months of therapy and then appeared to decrease over time. Because of the temporal nature of lipid changes, the 52-week, glyburide-controlled trial is most pertinent to assess long-term effects on lipids. At baseline, Week 26, and Week 52, mean LDL/HDL ratios were 3.1, 3.2, and 3.0, respectively, for rosiglitazone 4 mg twice daily. The corresponding values for glyburide were 3.2, 3.1, and 2.9. The differences in change from baseline between rosiglitazone and glyburide at Week 52 were statistically significant.
The pattern of LDL and HDL changes following therapy with rosiglitazone in combination with other hypoglycemic agents were generally similar to those seen with rosiglitazone in monotherapy. The changes in triglycerides during therapy with rosiglitazone were variable and were generally not statistically different from placebo or glyburide controls.
Pharmacokinetics
- Maximum plasma concentration (Cmax) and the area under the curve (AUC) of rosiglitazone increase in a dose-proportional manner over the therapeutic dose range (Table 8). The elimination half-life is 3 to 4 hours and is independent of dose.
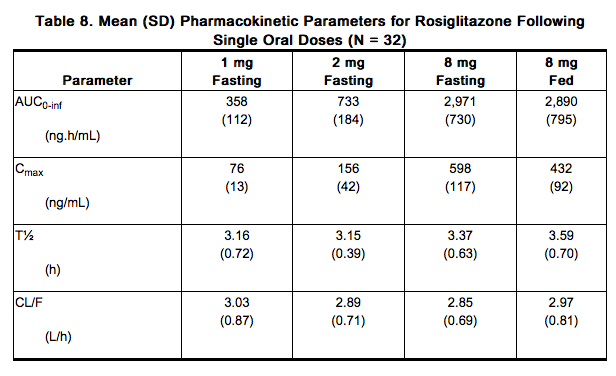
AUC = area under the curve; Cmax = maximum concentration; T½ = terminal half-life; CL/F = Oral clearance.
Absorption: The absolute bioavailability of rosiglitazone is 99%. Peak plasma concentrations are observed about 1 hour after dosing. Administration of rosiglitazone with food resulted in no change in overall exposure (AUC), but there was an approximately 28% decrease in Cmax and a delay in Tmax (1.75 hours). These changes are not likely to be clinically significant; therefore, rosiglitazone may be administered with or without food.
Distribution: The mean (CV%) oral volume of distribution (Vss/F) of rosiglitazone is approximately 17.6 (30%) liters, based on a population pharmacokinetic analysis. Rosiglitazone is approximately 99.8% bound to plasma proteins, primarily albumin.
Metabolism: Rosiglitazone is extensively metabolized with no unchanged drug excreted in the urine. The major routes of metabolism were N-demethylation and hydroxylation, followed by conjugation with sulfate and glucuronic acid. All the circulating metabolites are considerably less potent than parent and, therefore, are not expected to contribute to the insulin-sensitizing activity of rosiglitazone.
- In vitro data demonstrate that rosiglitazone is predominantly metabolized by Cytochrome P450 (CYP) isoenzyme 2C8, with CYP2C9 contributing as a minor pathway.
- Excretion: Following oral or intravenous administration of [14C]rosiglitazone maleate, approximately 64% and 23% of the dose was eliminated in the urine and in the feces, respectively. The plasma half-life of [14C]related material ranged from 103 to 158 hours.
Population Pharmacokinetics in Patients With type 2 diabetes: Population pharmacokinetic analyses from 3 large clinical trials including 642 men and 405 women with type 2 diabetes (aged 35 to 80 years) showed that the pharmacokinetics of rosiglitazone are not influenced by age, race, smoking, or alcohol consumption. Both oral clearance (CL/F) and oral steady-state volume of distribution (Vss/F) were shown to increase with increases in body weight. Over the weight range observed in these analyses (50 to 150 kg), the range of predicted CL/F and Vss/F values varied by <1.7-fold and <2.3-fold, respectively. Additionally, rosiglitazone CL/F was shown to be influenced by both weight and gender, being lower (about 15%) in female patients.
Special Populations: Geriatric: Results of the population pharmacokinetic analysis (n = 716 <65 years; n = 331 ≥65 years) showed that age does not significantly affect the pharmacokinetics of rosiglitazone.
Gender: Results of the population pharmacokinetics analysis showed that the mean oral clearance of rosiglitazone in female patients (n = 405) was approximately 6% lower compared with male patients of the same body weight (n = 642).
- As monotherapy and in combination with metformin, rosiglitazone improved glycemic control in both males and females. In metformin combination trials, efficacy was demonstrated with no gender differences in glycemic response.
- In monotherapy trials, a greater therapeutic response was observed in females; however, in more obese patients, gender differences were less evident. For a given body mass index (BMI), females tend to have a greater fat mass than males. Since the molecular target PPARγ is expressed in adipose tissues, this differentiating characteristic may account, at least in part, for the greater response to rosiglitazone in females. Since therapy should be individualized, no dose adjustments are necessary based on gender alone.
Hepatic Impairment: Unbound oral clearance of rosiglitazone was significantly lower in patients with moderate to severe liver disease (Child-Pugh Class B/C) compared with healthy subjects. As a result, unbound Cmax and AUC0-inf were increased 2- and 3-fold, respectively. Elimination half-life for rosiglitazone was about 2 hours longer in patients with liver disease, compared with healthy subjects.
Therapy with rosiglitazone should not be initiated if the patient exhibits clinical evidence of active liver disease or increased serum transaminase levels (ALT >2.5X upper limit of normal) at baseline.
Pediatric: Pharmacokinetic parameters of rosiglitazone in pediatric patients were established using a population pharmacokinetic analysis with sparse data from 96 pediatric patients in a single pediatric clinical trial including 33 males and 63 females with ages ranging from 10 to 17 years (weights ranging from 35 to 178.3 kg). Population mean CL/F and V/F of rosiglitazone were 3.15 L/h and 13.5 L, respectively. These estimates of CL/F and V/F were consistent with the typical parameter estimates from a prior adult population analysis.
Renal Impairment: There are no clinically relevant differences in the pharmacokinetics of rosiglitazone in patients with mild to severe renal impairment or in hemodialysis-dependent patients compared with subjects with normal renal function. No dosage adjustment is therefore required in such patients receiving rosiglitazone. Since metformin is contraindicated in patients with renal impairment, coadministration of metformin with rosiglitazone is contraindicated in these patients.
Race: Results of a population pharmacokinetic analysis including subjects of Caucasian, black, and other ethnic origins indicate that race has no influence on the pharmacokinetics of rosiglitazone.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: A 2-year carcinogenicity study was conducted in Charles River CD-1 mice at doses of 0.4, 1.5, and 6 mg/kg/day in the diet (highest dose equivalent to approximately 12 times human AUC at the maximum recommended human daily dose). Sprague-Dawley rats were dosed for 2 years by oral gavage at doses of 0.05, 0.3, and 2 mg/kg/day (highest dose equivalent to approximately 10 and 20 times human AUC at the maximum recommended human daily dose for male and female rats, respectively).
- Rosiglitazone was not carcinogenic in the mouse. There was an increase in incidence of adipose hyperplasia in the mouse at doses ≥1.5 mg/kg/day (approximately 2 times human AUC at the maximum recommended human daily dose). In rats, there was a significant increase in the incidence of benign adipose tissue tumors (lipomas) at doses ≥0.3 mg/kg/day (approximately 2 times human AUC at the maximum recommended human daily dose). These proliferative changes in both species are considered due to the persistent pharmacological overstimulation of adipose tissue.
Mutagenesis: Rosiglitazone was not mutagenic or clastogenic in the in vitro bacterial assays for gene mutation, the in vitro chromosome aberration test in human lymphocytes, the in vivo mouse micronucleus test, and the in vivo/in vitro rat UDS assay. There was a small (about 2-fold) increase in mutation in the in vitro mouse lymphoma assay in the presence of metabolic activation. Impairment of Fertility: Rosiglitazone had no effects on mating or fertility of male rats given up to 40 mg/kg/day (approximately 116 times human AUC at the maximum recommended human daily dose). Rosiglitazone altered estrous cyclicity (2 mg/kg/day) and reduced fertility (40 mg/kg/day) of female rats in association with lower plasma levels of progesterone and estradiol (approximately 20 and 200 times human AUC at the maximum recommended human daily dose, respectively). No such effects were noted at 0.2 mg/kg/day (approximately 3 times human AUC at the maximum recommended human daily dose). In juvenile rats dosed from 27 days of age through to sexual maturity (at up to 40 mg/kg/day), there was no effect on male reproductive performance, or on estrous cyclicity, mating performance or pregnancy incidence in females (approximately 68 times human AUC at the maximum recommended human daily dose). In monkeys, rosiglitazone (0.6 and 4.6 mg/kg/day; approximately 3 and 15 times human AUC at the maximum recommended human daily dose, respectively) diminished the follicular phase rise in serum estradiol with consequential reduction in the luteinizing hormone surge, lower luteal phase progesterone levels, and amenorrhea. The mechanism for these effects appears to be direct inhibition of ovarian steroidogenesis.
Animal Toxicology
- Heart weights were increased in mice (3 mg/kg/day), rats (5 mg/kg/day), and dogs (2 mg/kg/day) with rosiglitazone treatments (approximately 5, 22, and 2 times human AUC at the maximum recommended human daily dose, respectively). Effects in juvenile rats were consistent with those seen in adults. Morphometric measurement indicated that there was hypertrophy in cardiac ventricular tissues, which may be due to increased heart work as a result of plasma volume expansion.
Clinical Studies
Monotherapy
- In clinical trials, treatment with rosiglitazone resulted in an improvement in glycemic control, as measured by FPG and HbA1c, with a concurrent reduction in insulin and C-peptide. Postprandial glucose and insulin were also reduced. This is consistent with the mechanism of action of rosiglitazone as an insulin sensitizer.
- The maximum recommended daily dose is 8 mg. Dose-ranging trials suggested that no additional benefit was obtained with a total daily dose of 12 mg.
Short-term Clinical Trials: A total of 2,315 patients with type 2 diabetes, previously treated with diet alone or antidiabetic medication(s), were treated with rosiglitazone as monotherapy in 6 double-blind trials, which included two 26-week, placebo-controlled trials; one 52-week, glyburide-controlled trial; and 3 placebo-controlled, dose-ranging trials of 8 to 12 weeks’ duration. Previous antidiabetic medication(s) were withdrawn and patients entered a 2- to 4-week placebo run-in period prior to randomization.
- Two 26-week, double-blind, placebo-controlled trials, in patients with type 2 diabetes (n = 1,401) with inadequate glycemic control [mean baseline FPG approximately 228 mg/dL (101 to 425 mg/dL) and mean baseline HbA1c 8.9% (5.2% to 16.2%)], were conducted. Treatment with rosiglitazone produced statistically significant improvements in FPG and HbA1c compared with baseline and relative to placebo. Data from one of these trials are summarized in Table 9.
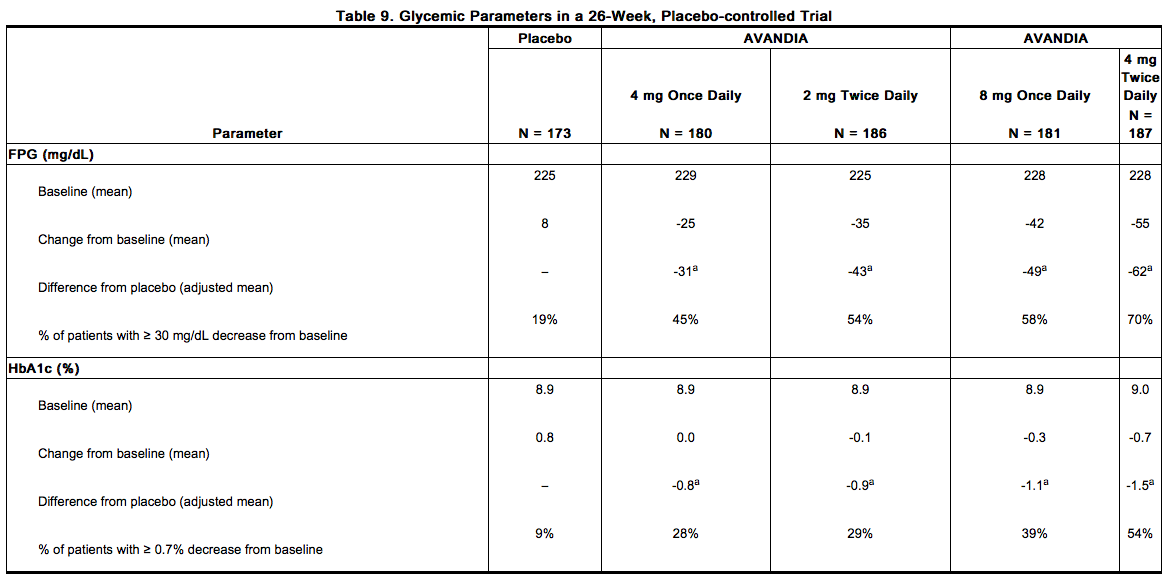
- When administered at the same total daily dose, rosiglitazone was generally more effective in reducing FPG and HbA1c when administered in divided doses twice daily compared with once-daily doses. However, for HbA1c, the difference between the 4 mg once-daily and 2 mg twice-daily doses was not statistically significant.
Long-term Clinical Trials: Long-term maintenance of effect was evaluated in a 52-week, double-blind, glyburide-controlled trial in patients with type 2 diabetes. Patients were randomized to treatment with rosiglitazone 2 mg twice daily (N = 195) or rosiglitazone 4 mg twice daily (N = 189) or glyburide (N = 202) for 52 weeks. Patients receiving glyburide were given an initial dosage of either 2.5 mg/day or 5.0 mg/day. The dosage was then titrated in 2.5-mg/day increments over the next 12 weeks, to a maximum dosage of 15.0 mg/day in order to optimize glycemic control. Thereafter, the glyburide dose was kept constant. The median titrated dose of glyburide was 7.5 mg. All treatments resulted in a statistically significant improvement in glycemic control from baseline (Figure 3 and Figure 4). At the end of Week 52, the reduction from baseline in FPG and HbA1c was -40.8 mg/dL and -0.53% with rosiglitazone 4 mg twice daily; -25.4 mg/dL and -0.27% with rosiglitazone 2 mg twice daily; and -30.0 mg/dL and -0.72% with glyburide. For HbA1c, the difference between rosiglitazone 4 mg twice daily and glyburide was not statistically significant at Week 52. The initial fall in FPG with glyburide was greater than with rosiglitazone; however, this effect was less durable over time. The improvement in glycemic control seen with rosiglitazone 4 mg twice daily at Week 26 was maintained through Week 52 of the trial.
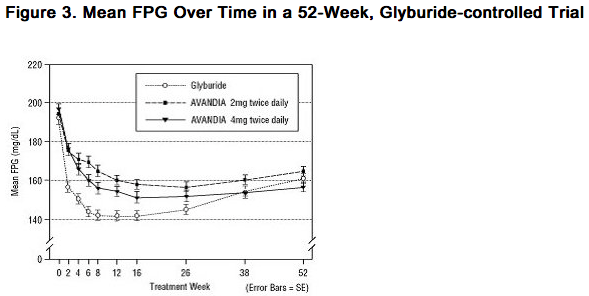
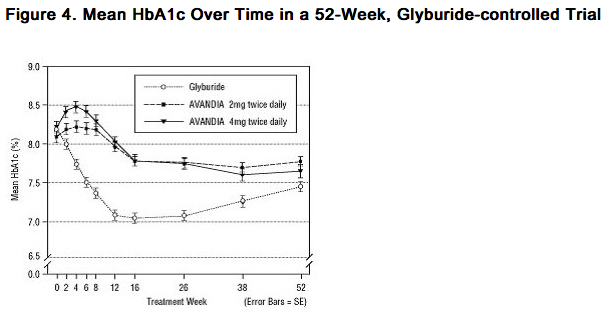
- Hypoglycemia was reported in 12.1% of glyburide-treated patients versus 0.5% (2 mg twice daily) and 1.6% (4 mg twice daily) of patients treated with rosiglitazone. The improvements in glycemic control were associated with a mean weight gain of 1.75 kg and 2.95 kg for patients treated with 2 mg and 4 mg twice daily of rosiglitazone, respectively, versus 1.9 kg in glyburide-treated patients. In patients treated with rosiglitazone, C-peptide, insulin, pro-insulin, and pro-insulin split products were significantly reduced in a dose-ordered fashion, compared with an increase in the glyburide-treated patients.
A Diabetes Outcome Progression Trial (ADOPT) was a multicenter, double-blind, controlled trial (N = 4,351) conducted over 4 to 6 years to compare the safety and efficacy of rosiglitazone, metformin, and glyburide monotherapy in patients recently diagnosed with type 2 diabetes mellitus (≤3 years) inadequately controlled with diet and exercise. The mean age of patients in this trial was 57 years and the majority of patients (83%) had no known history of cardiovascular disease. The mean baseline FPG and HbA1c were 152 mg/dL and 7.4%, respectively. Patients were randomized to receive either rosiglitazone 4 mg once daily, glyburide 2.5 mg once daily, or metformin 500 mg once daily, and doses were titrated to optimal glycemic control up to a maximum of 4 mg twice daily for Rosiglitazone, 7.5 mg twice daily for glyburide, and 1,000 mg twice daily for metformin. The primary efficacy outcome was time to consecutive FPG >180 mg/dL after at least 6 weeks of treatment at the maximum tolerated dose of study medication or time to inadequate glycemic control, as determined by an independent adjudication committee.
- The cumulative incidence of the primary efficacy outcome at 5 years was 15% with rosiglitazone, 21% with metformin, and 34% with glyburide (HR 0.68 [95% CI: 0.55, 0.85] versus metformin, HR 0.37 [95% CI: 0.30, 0.45] versus glyburide).
Cardiovascular and adverse event data (including effects on body weight and bone fracture) from ADOPT for rosiglitazone, metformin, and glyburide are described in Warnings and Precautions and adverse reactions respectively. As with all medications, efficacy results must be considered together with safety information to assess the potential benefit and risk for an individual patient.
Combination With Metformin or Sulfonylurea
- The addition of rosiglitazone to either metformin or sulfonylurea resulted in significant reductions in hyperglycemia compared with either of these agents alone. These results are consistent with an additive effect on glycemic control when rosiglitazone is used as combination therapy.
Combination With Metformin: A total of 670 patients with type 2 diabetes participated in two 26-week, randomized, double-blind, placebo/active-controlled trials designed to assess the efficacy of rosiglitazone in combination with metformin. Rosiglitazone, administered in either once-daily or twice-daily dosing regimens, was added to the therapy of patients who were inadequately controlled on a maximum dose (2.5 grams/day) of metformin.
- In one trial, patients inadequately controlled on 2.5 grams/day of metformin (mean baseline FPG 216 mg/dL and mean baseline HbA1c 8.8%) were randomized to receive 4 mg of rosiglitazone once daily, 8 mg of rosiglitazone once daily, or placebo in addition to metformin. A statistically significant improvement in FPG and HbA1c was observed in patients treated with the combinations of metformin and 4 mg of rosiglitazone once daily and 8 mg of rosiglitazone once daily, versus patients continued on metformin alone (Table 10).
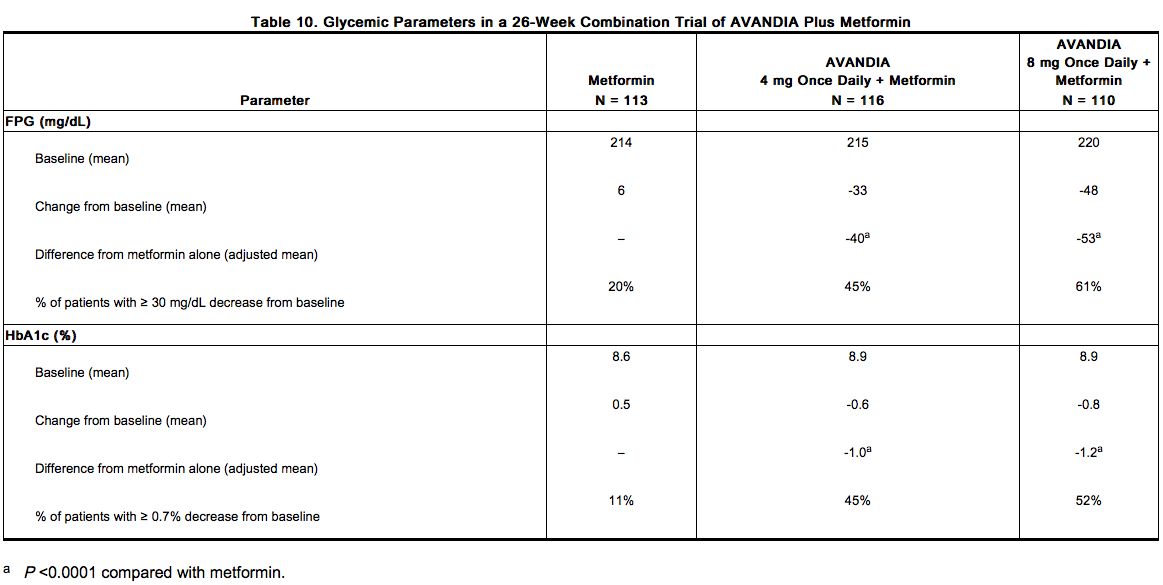
- In a second 26-week trial, patients with type 2 diabetes inadequately controlled on 2.5 grams/day of metformin who were randomized to receive the combination of rosiglitazone 4 mg twice daily and metformin (N = 105) showed a statistically significant improvement in glycemic control with a mean treatment effect for FPG of -56 mg/dL and a mean treatment effect for HbA1c of -0.8% over metformin alone. The combination of metformin and rosiglitazone resulted in lower levels of FPG and HbA1c than either agent alone.
- Patients who were inadequately controlled on a maximum dose (2.5 grams/day) of metformin and who were switched to monotherapy with rosiglitazone demonstrated loss of glycemic control, as evidenced by increases in FPG and HbA1c. In this group, increases in LDL and VLDL were also seen.
Combination With a Sulfonylurea: A total of 3,457 patients with type 2 diabetes participated in ten 24- to 26-week randomized, double-blind, placebo/active-controlled trials and one 2-year double-blind, active-controlled trial in elderly patients designed to assess the efficacy and safety of rosiglitazone in combination with a sulfonylurea. Rosiglitazone 2 mg, 4 mg, or 8 mg daily was administered, either once daily (3 trials) or in divided doses twice daily (7 trials), to patients inadequately controlled on a submaximal or maximal dose of sulfonylurea.
- In these trials, the combination of rosiglitazone 4 mg or 8 mg daily (administered as single- or twice-daily divided doses) and a sulfonylurea significantly reduced FPG and HbA1c compared with placebo plus sulfonylurea or further up-titration of the sulfonylurea. Table 11 shows pooled data for 8 trials in which rosiglitazone added to sulfonylurea was compared with placebo plus sulfonylurea.
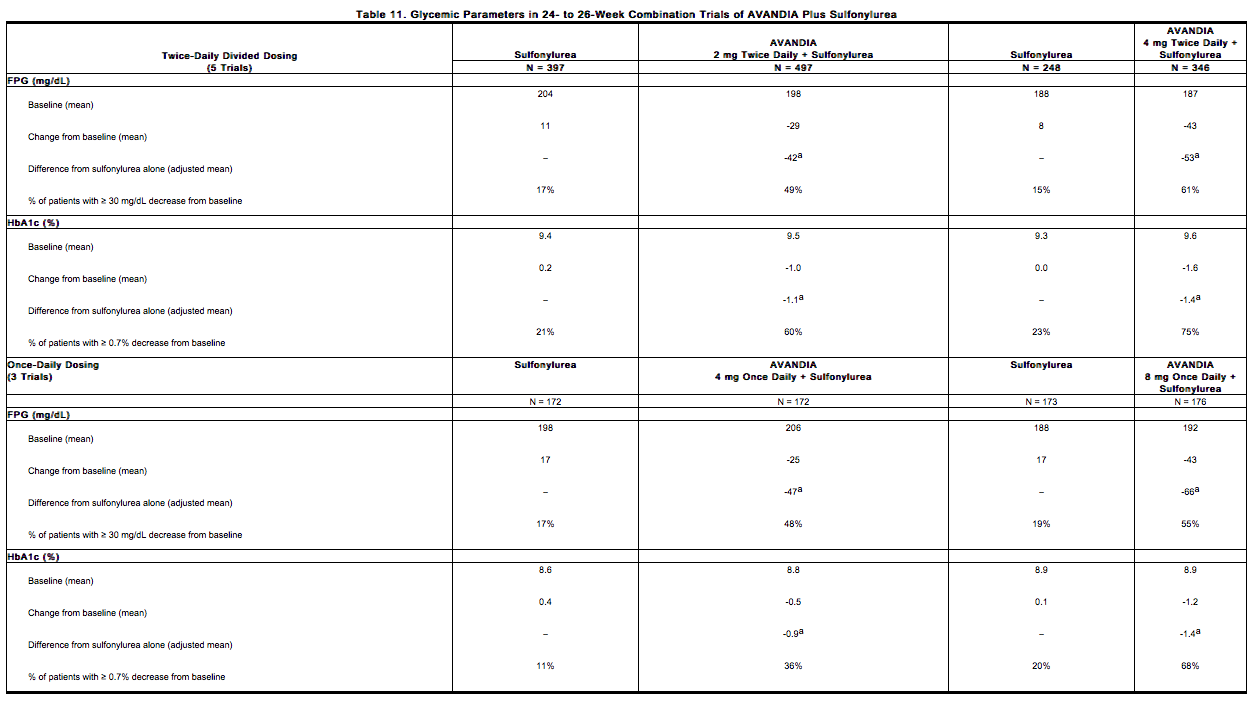
- One of the 24- to 26-week trials included patients who were inadequately controlled on maximal doses of glyburide and switched to 4 mg of rosiglitazone daily as monotherapy; in this group, loss of glycemic control was demonstrated, as evidenced by increases in FPG and HbA1c.
In a 2-year, double-blind trial, elderly patients (aged 59 to 89 years) on half-maximal sulfonylurea (glipizide 10 mg twice daily) were randomized to the addition of rosiglitazone (n = 115, 4 mg once daily to 8 mg as needed) or to continued up-titration of glipizide (n = 110), to a maximum of 20 mg twice daily. Mean baseline FPG and HbA1c were 157 mg/dL and 7.72%, respectively, for the arm receiving rosiglitazone plus glipizide and 159 mg/dL and 7.65%, respectively, for the glipizide up-titration arm. Loss of glycemic control (FPG ≥180 mg/dL) occurred in a significantly lower proportion of patients (2%) on rosiglitazone plus glipizide compared with patients in the glipizide up-titration arm (28.7%). About 78% of the patients on combination therapy completed the 2 years of therapy while only 51% completed on glipizide monotherapy. The effect of combination therapy on FPG and HbA1c was durable over the 2-year trial period, with patients achieving a mean of 132 mg/dL for FPG and a mean of 6.98% for HbA1c compared with no change on the glipizide arm.
Combination With Sulfonylurea Plus Metformin
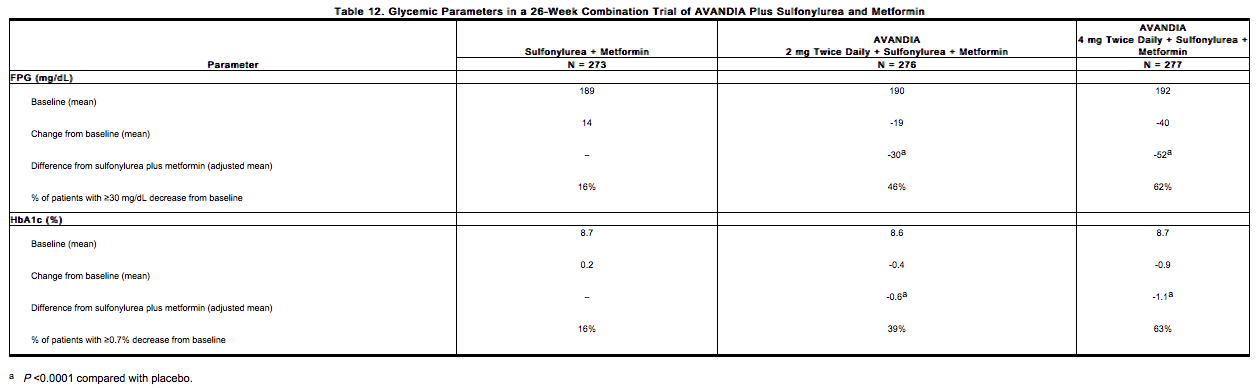
- In two 24- to 26-week, double-blind, placebo-controlled trials designed to assess the efficacy and safety of rosiglitazone in combination with sulfonylurea plus metformin, rosiglitazone 4 mg or 8 mg daily, was administered in divided doses twice daily, to patients inadequately controlled on submaximal (10 mg) and maximal (20 mg) doses of glyburide and maximal dose of metformin (2 g/day). A statistically significant improvement in FPG and HbA1c was observed in patients treated with the combinations of sulfonylurea plus metformin and 4 mg of rosiglitazone and 8 mg of rosiglitazone versus patients continued on sulfonylurea plus metformin, as shown in Table 12.
How Supplied
There is limited information regarding Rosiglitazone How Supplied in the drug label.
Storage
There is limited information regarding Rosiglitazone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Rosiglitazone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Rosiglitazone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Rosiglitazone Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-rosiglitazone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
AVANDIA
Look-Alike Drug Names
Rosiglitazone - Coumadin Rosiglitazone - Prandin[6]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Choi D, Kim SK, Choi SH, Ko YG, Ahn CW, Jang Y; et al. (2004). "Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with [[type 2 diabetes]]". Diabetes Care. 27 (11): 2654–60. PMID 15505001. URL–wikilink conflict (help)
- ↑ Lin SH, Lin YF, Kuo SW, Hsu YJ, Hung YJ (2003). "Rosiglitazone improves glucose metabolism in nondiabetic uremic patients on CAPD". Am J Kidney Dis. 42 (4): 774–80. PMID 14520628.
- ↑ Wang TD, Chen WJ, Lin JW, Chen MF, Lee YT (2004). "Effects of rosiglitazone on endothelial function, C-reactive protein, and components of the metabolic syndrome in nondiabetic patients with the metabolic syndrome". Am J Cardiol. 93 (3): 362–5. doi:10.1016/j.amjcard.2003.10.022. PMID 14759393.
- ↑ Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L; et al. (2008). "Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial". Gastroenterology. 135 (1): 100–10. doi:10.1053/j.gastro.2008.03.078. PMID 18503774.
- ↑ Ghazeeri G, Kutteh WH, Bryer-Ash M, Haas D, Ke RW (2003). "Effect of rosiglitazone on spontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome". Fertil Steril. 79 (3): 562–6. PMID 12620440.
- ↑ "https://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Rosiglitazone |Pill Name=No_image.jpg |Drug Name=Rosiglitazone 2 MG Oral Tablet |Pill Ingred=hypromellose 2910 (3 mpa.s), lactose monohydrate / magnesium stearate / cellulose, microcrystalline / polyethylene glycol 3000 / sodium starch glycolate type a potato / titanium dioxide / triacetin / talc / ferric oxide red / ferric oxide yellow,|+sep=; |Pill Imprint=SB;2 |Pill Dosage=2 mg |Pill Color=Pink|+sep=; |Pill Shape=FreeForm |Pill Size (mm)=10.00 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=0173-0834
}}
{{#subobject:
|Page Name=Rosiglitazone |Pill Name=No_image.jpg |Drug Name=Rosiglitazone 4 MG Oral Tablet |Pill Ingred=hypromellose 2910 (3 mpa.s), lactose monohydrate / magnesium stearate / cellulose, microcrystalline / polyethylene glycol 3000 / sodium starch glycolate type a potato / titanium dioxide / triacetin / talc / ferric oxide red / ferric oxide yellow,|+sep=; |Pill Imprint=SB;4 |Pill Dosage=4 mg |Pill Color=Orange|+sep=; |Pill Shape=FreeForm |Pill Size (mm)=10.00 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=0173-0835
}}
{{#subobject:
|Page Name=Rosiglitazone |Pill Name=No_image.jpg |Drug Name=Rosiglitazone 8 MG Oral Tablet |Pill Ingred=hypromellose 2910 (3 mpa.s), lactose monohydrate / magnesium stearate / cellulose, microcrystalline / polyethylene glycol 3000 / sodium starch glycolate type a potato / titanium dioxide / triacetin / talc / ferric oxide red / ferric oxide yellow,|+sep=; |Pill Imprint=SB;8 |Pill Dosage=4 mg |Pill Color=Red|+sep=; |Pill Shape=FreeForm |Pill Size (mm)=12.00 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=0173-0836
}}
{{#subobject:
|Page Name=Rosiglitazone |Pill Name=No_image.jpg |Drug Name=Rosiglitazone 2 MG Oral Tablet |Pill Ingred=hypromellose 2910 (3 mpa.s), lactose monohydrate / magnesium stearate / cellulose, microcrystalline / polyethylene glycol 3000 / sodium starch glycolate type a potato / titanium dioxide / triacetin / talc / ferric oxide red / ferric oxide yellow,|+sep=; |Pill Imprint=GSK;2 |Pill Dosage=4 mg |Pill Color=Pink|+sep=; |Pill Shape=FreeForm |Pill Size (mm)=10.00 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=0173-0861
}}
{{#subobject:
|Page Name=Rosiglitazone |Pill Name=No_image.jpg |Drug Name=Rosiglitazone 4 MG Oral Tablet |Pill Ingred=hypromellose 2910 (3 mpa.s), lactose monohydrate / magnesium stearate / cellulose, microcrystalline / polyethylene glycol 3000 / sodium starch glycolate type a potato / titanium dioxide / triacetin / talc / ferric oxide red / ferric oxide yellow,|+sep=; |Pill Imprint=GSK;4 |Pill Dosage=4 mg |Pill Color=Orange|+sep=; |Pill Shape=FreeForm |Pill Size (mm)=10.00 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=0173-0863
}}
{{#subobject:
|Page Name=Rosiglitazone |Pill Name=No_image.jpg |Drug Name=Rosiglitazone 8 MG Oral Tablet |Pill Ingred=hypromellose 2910 (3 mpa.s), lactose monohydrate / magnesium stearate / cellulose, microcrystalline / polyethylene glycol 3000 / sodium starch glycolate type a potato / titanium dioxide / triacetin / talc / ferric oxide red / ferric oxide yellow,|+sep=; |Pill Imprint=SB;4 |Pill Dosage=4 mg |Pill Color=Red|+sep=; |Pill Shape=FreeForm |Pill Size (mm)=12.00 |Pill Scoring=1 |Pill Image= |Drug Author=GlaxoSmithKline LLC |NDC=0173-0864
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_label_01.jpg
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_label_02.jpg
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_label_03.jpg
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_label_04.jpg
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_label_05.jpg
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_label_06.jpg
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_panel_01.png
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_panel_02.png
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_panel_03.png
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_panel_04.png
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_panel_05.png
}}
{{#subobject:
|Label Page=Rosiglitazone |Label Name=Rosglitazone_panel_06.png
}}