Riociguat: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 150: | Line 150: | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=* It is not known if Adempas is present in human milk. Riociguat or its metabolites were present in the milk of rats. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from riociguat, discontinue nursing or Adempas. | |useInNursing=* It is not known if Adempas is present in human milk. Riociguat or its metabolites were present in the milk of rats. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from riociguat, discontinue nursing or Adempas. | ||
|useInPed=* Safety and effectiveness of Adempas in pediatric patients have not been established. | |useInPed=* Safety and effectiveness of Adempas in pediatric patients have not been established. | ||
|useInGeri=* Of the total number of subjects in clinical studies of Adempas, 23% were 65 and over, and 6% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | |useInGeri=* Of the total number of subjects in clinical studies of Adempas, 23% were 65 and over, and 6% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | ||
| Line 163: | Line 163: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |administration=* Oral | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
| Line 170: | Line 168: | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
|overdose=* In cases of overdose, blood pressure should be closely monitored and supported as appropriate. Based on extensive plasma protein binding, riociguat is not expected to be dialyzable. | |overdose=* In cases of overdose, blood pressure should be closely monitored and supported as appropriate. Based on extensive plasma protein binding, riociguat is not expected to be dialyzable. | ||
|drugBox=<!--Mechanism of Action--> | |drugBox=<!--Mechanism of Action--> | ||
| Line 194: | Line 190: | ||
* Each round film-coated tablet contains 0.5 mg (1.0, 1.5, 2.0, 2.5 mg) riociguat. The inactive ingredients are cellulose microcrystalline, crospovidone, hypromellose 5cP, lactose monohydrate, magnesium stearate, sodium laurylsulfate, hydroxypropylcellulose, hypromellose 3cP, propylene glycol, and titanium dioxide. Adempas 1, 1.5, 2 and 2.5 mg tablets contain, in addition, ferric oxide yellow. Adempas 2 and 2.5 mg tablets contain, in addition, ferric oxide red.. | * Each round film-coated tablet contains 0.5 mg (1.0, 1.5, 2.0, 2.5 mg) riociguat. The inactive ingredients are cellulose microcrystalline, crospovidone, hypromellose 5cP, lactose monohydrate, magnesium stearate, sodium laurylsulfate, hydroxypropylcellulose, hypromellose 3cP, propylene glycol, and titanium dioxide. Adempas 1, 1.5, 2 and 2.5 mg tablets contain, in addition, ferric oxide yellow. Adempas 2 and 2.5 mg tablets contain, in addition, ferric oxide red.. | ||
|PD=* There is a direct relationship between riociguat plasma concentration and hemodynamic parameters such as systemic vascular resistance, systolic blood pressure, pulmonary vascular resistance (PVR), and cardiac output. | |||
* Hemodynamic parameters were assessed in CTEPH patients in CHEST-1. Right heart catheterization was performed at the beginning and the end of the study period in 233 patients. A statistically significant reduction of PVR (-246 dyn*s*cm-5) was shown in the Adempas group vs. placebo. Improvements in other hemodynamic parameters (not pre-specified as endpoints) are displayed in Table 2 below. | |||
Hemodynamic parameters were assessed in CTEPH patients in CHEST-1 | |||
[[File:Rociguat table2.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | [[File:Rociguat table2.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
| Line 222: | Line 217: | ||
''Acetylsalicylic Acid'': Concomitant use of riociguat and aspirin did not affect bleeding time or platelet aggregation | ''Acetylsalicylic Acid'': Concomitant use of riociguat and aspirin did not affect bleeding time or platelet aggregation | ||
|PK=* Riociguat pharmacokinetics are dose proportional from 0.5 to 2.5 mg. Inter-individual variability of riociguat exposure (AUC) across all doses is approximately 60%, and within-subject variability is approximately 30%. | |||
'''Absorption and distribution''' | |||
* The absolute bioavailability of riociguat is about 94%. Peak plasma riociguat concentrations were observed within 1.5 hours after tablet intake. Food does not affect the bioavailability of riociguat. | |||
* The volume of distribution at steady state is approximately 30 L. Plasma protein binding in humans is approximately 95%, with serum albumin and α1–acidic glycoprotein being the main binding components. | |||
* Riociguat is a substrate of P-gp and BCRP. | |||
'''Metabolism and excretion''' | |||
* Riociguat is mainly cleared by metabolism by CYP1A1, CYP3A, CYP2C8 and CYP2J2. Formation of the major active metabolite, M1, is catalyzed by CYP1A1, which is inducible by polycyclic aromatic hydrocarbons such as those present in cigarette smoke. M1 is further metabolized to the inactive N-glucuronide. Plasma concentrations of M1 in patients with PAH are about half those for riociguat. | |||
* Following oral administration of radiolabeled riociguat in healthy individuals, about 40 and 53% of the total radioactivity was recovered in urine and feces, respectively. There appears to be considerable variability in the proportion of metabolites and unchanged riociguat excreted, but metabolites were the major components of the dose excreted in most individuals. | |||
* Average systemic clearance of riociguat was about 1.8 L/h in patients with PAH and about 3.4 L/h in healthy subjects. The terminal elimination half-life is about 12 hours in patients and 7 hours in healthy subjects. | |||
''Specific Populations'': The effect of intrinsic factors on riociguat and M1 are shown below in Figure 1. There are no clinically relevant effects of age, sex, weight, or race/ethnicity on the pharmacokinetics of riociguat or M1. No dose adjustment is warranted. | |||
[[File:Rociguat fig1.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
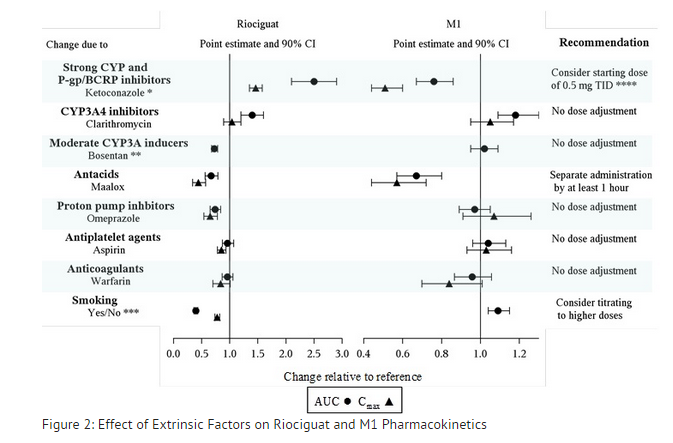
''Drug interactions'': The effect of other extrinsic factors on riociguat and M1 were studied in healthy subjects and are shown in Figure 2 | |||
[[File:Rociguat fig2.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*HIV protease inhibitors are strong CYP3A inhibitors and may increase riociguat plasma concentrations to levels similar to those seen with ketoconazole. ** AUC only, estimated using population pharmacokinetics methods *** AUC only for metabolite, estimated using population pharmacokinetics methods. **** Monitor for signs and symptoms of hypotension on initiation and on treatment with strong CYP and P-gp/BCRP inhibitors. | |||
''Strong CYP3A inducers'': Data are not available to inform dosing of riociguat when strong CYP3A inducers are co-administered. | |||
''Effects of Riociguat on other Drugs'': Riociguat did not affect the pharmacokinetics of midazolam, warfarin, or sildenafil. | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | ||
Revision as of 14:05, 27 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
* Do not administer Adempas to a pregnant female because it may cause fetal harm.
|
Overview
Riociguat is a {{{drugClass}}} that is FDA approved for the treatment of persistent,recurrent chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include headache, dyspepsia,gastritis, dizziness, nausea, diarrhea, hypotension, vomiting, anemia, gastroesophageal reflux, and constipation..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Chronic-Thromboembolic Pulmonary Hypertension
- Adempas is indicated for the treatment of adults with persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH), (WHO Group 4) after surgical treatment, or inoperable CTEPH, to improve exercise capacity and WHO functional class.
Pulmonary Arterial Hypertension
- Adempas is indicated for the treatment of adults with pulmonary arterial hypertension (PAH), (WHO Group 1), to improve exercise capacity, WHO functional class and to delay clinical worsening.
- Efficacy was shown in patients on Adempas monotherapy or in combination with endothelin receptor antagonists or prostanoids. Studies establishing effectiveness included predominately patients with WHO functional class II–III and etiologies of idiopathic or heritable PAH (61%) or PAH associated with connective tissue diseases (25%).
Dosage
Recommended Dosage in Adult Patients
- The recommended starting dosage is 1 mg taken 3 times a day. For patients who may not tolerate the hypotensive effect of Adempas, consider a starting dose of 0.5 mg taken three times a day. If systolic blood pressure remains greater than 95 mmHg and the patient has no signs or symptoms of hypotension, up-titrate the dose by 0.5 mg taken three times a day. Dose increases should be no sooner than 2 weeks apart. The dose can be increased to the highest tolerated dosage, up to a maximum of 2.5 mg taken three times a day. If at any time, the patient has symptoms of hypotension, decrease the dosage by 0.5 mg taken three times a day.
Dosage Interruption
- If a dose is missed, advise patients to continue with the next regularly scheduled dose.
- In case Adempas is interrupted for 3 days or more, re-titrate Adempas.
Pregnancy Testing in Females of Reproductive Potential
- Obtain pregnancy tests prior to initiation and monthly during treatment.
Use in Patients who Smoke
- Consider titrating to dosages higher than 2.5 mg three times a day, if tolerated, in patients who smoke. A dose decrease may be required in patients who stop smoking.
Strong CYP and P-gp/BCRP Inhibitors
- Consider a starting dose of 0.5 mg, three times a day when initiating Adempas in patients receiving strong cytochrome P450 (CYP) and P-glycoprotein/breast cancer resistance protein (P-gp/BCRP) inhibitors such as azole antimycotics (for example, ketoconazole, itraconazole) or HIV protease inhibitors (for example, ritonavir). Monitor for signs and symptoms of hypotension on initiation and on treatment with strong CYP and P-gp/BCRP inhibitors.
DOSAGE FORMS AND STRENGTHS
- Tablets: film-coated, round, bi-convex:
- 0.5 mg, white, with “BAYER” cross on one side and “0.5” and “R” on the other side
- 1 mg, pale-yellow, with “BAYER” cross on one side and “1” and “R” on the other side
- 1.5 mg, yellow-orange, with “BAYER” cross on one side and “1.5” and “R” on the other side
- 2 mg, pale orange, with “BAYER” cross on one side and “2” and “R” on the other side
- 2.5 mg, red-orange, with “BAYER” cross on one side and “2.5” and “R” on the other side
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Riociguat in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Riociguat in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There is limited information regarding FDA-Labeled Use of Riociguat in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Riociguat in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Riociguat in pediatric patients.
Contraindications
Pregnancy
- Adempas may cause fetal harm when administered to a pregnant woman. Adempas is contraindicated in females who are pregnant. Adempas was consistently shown to have teratogenic effects when administered to animals. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus .
Nitrates and Nitric Oxide Donors
- Co-administration of Adempas with nitrates or nitric oxide donors (such as amyl nitrite) in any form is contraindicated.
Phosphodiesterase Inhibitors
- Concomitant administration of Adempas with specific PDE-5 inhibitors (such as sildenafil, tadalafil, or vardenafil) or nonspecific PDE inhibitors (such as dipyridamole or theophylline) is contraindicated .
Warnings
|
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
* Do not administer Adempas to a pregnant female because it may cause fetal harm.
|
Embryo-Fetal Toxicity
- Adempas may cause fetal harm when administered during pregnancy and is contraindicated for use in women who are pregnant. In females of reproductive potential, exclude pregnancy prior to initiation of therapy, advise use of acceptable contraception and obtain monthly pregnancy tests. For Female, Adempas is only available through a restricted program under the Adempas REMS Program.
Adempas REMS Program
- Females can only receive Adempas through the Adempas Risk Evaluation and Mitigation Strategy (REMS) Program, a restricted distribution program.
- Important requirements of the Adempas REMS Program include the following:
- Prescribers must be certified with the program by enrolling and completing training.
- All females, regardless of reproductive potential, must enroll in the Adempas REMS Program prior to initiating Adempas. Male patients are not enrolled in the Adempas REMS Program.
- Female patients of reproductive potential must comply with the pregnancy testing and contraception requirements.
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive Adempas.
Hypotension
- Adempas reduces blood pressure. Consider the potential for symptomatic hypotension or ischemia in patients with hypovolemia, severe left ventricular outflow obstruction, resting hypotension, autonomic dysfunction, or concomitant treatment with antihypertensives or strong CYP and P-gp/BCRP inhibitors. Consider a dose reduction if patient develops signs or symptoms of hypotension.
Bleeding
- In the placebo-controlled clinical trials, serious bleeding occurred in 2.4% of patients taking Adempas compared to 0% of placebo patients. Serious hemoptysis occurred in 5 (1%) patients taking Adempas compared to 0 placebo patients, including one event with fatal outcome. Serious hemorrhagic events also included 2 patients with vaginal hemorrhage, 2 with catheter site hemorrhage, and 1 each with subdural hematoma, hematemesis, and intra-abdominal hemorrhage.
Pulmonary Veno-Occlusive Disease
- Pulmonary vasodilators may significantly worsen the cardiovascular status of patients with pulmonary veno-occlusive disease (PVOD). Therefore, administration of Adempas to such patients is not recommended. Should signs of pulmonary edema occur, the possibility of associated PVOD should be considered and, if confirmed, discontinue treatment with Adempas.
Adverse Reactions
Clinical Trials Experience
- The following serious adverse reactions are discussed elsewhere in the labeling:
- Embryo-Fetal Toxicity
- Hypotension
- Bleeding
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety data described below reflect exposure to Adempas in two, randomized, double blind, placebo-controlled trials in patients with inoperable or recurrent/persistent CTEPH (CHEST-1) and treatment naive or pre-treated PAH patients (PATENT-1). The population (Adempas: n = 490; Placebo: n = 214) was between the age of 18 and 80 years.
- The safety profile of Adempas in patients with inoperable or recurrent/persistent CTEPH (CHEST-1) and treatment naive or pre-treated PAH (PATENT-1) were similar. Therefore, adverse drug reactions (ADRs) identified from the 12 and 16 week placebo-controlled trials for PAH and CTEPH respectively were pooled, and those occurring more frequently on Adempas than placebo (≥3%) are displayed in Table 1 below. Most adverse reactions in Table 1 can be ascribed to the vasodilatory mechanism of action of Adempas.
- The overall rates of discontinuation due to an adverse event in the pivotal placebo-controlled trials were 2.9% for Adempas and 5.1% for placebo (pooled data).

Other events that were seen more frequently in Adempas compared to placebo and potentially related to treatment were: palpitations, nasal congestion, epistaxis, dysphagia, abdominal distension and peripheral edema. With longer observation in uncontrolled long-term extension studies the safety profile was similar to that observed in the placebo controlled phase 3 trials.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Riociguat in the drug label.
Drug Interactions
Pharmacodynamic Interactions with Adempas
Nitrates: Co-administration of Adempas with nitrates or nitric oxide donors (such as amyl nitrite) in any form is contraindicated because of hypotension.
PDE Inhibitors: Co-administration of Adempas with specific PDE-5 inhibitors (such as sildenafil, tadalafil, or vardenafil) and nonspecific PDE inhibitors (such as dipyridamole or theophylline), is contraindicated because of hypotension. Clinical experience with co-administration of Adempas and other phosphodiesterase inhibitors (for example, milrinone, cilostazole, roflumilast) is limited.
Pharmacokinetic Interactions with Adempas
Smoking: Plasma concentrations in smokers are reduced by 50-60% compared to nonsmokers. Based on pharmacokinetic modeling, for patients who are smokers, doses higher than 2.5 mg three times a day may be considered in order to match exposure seen in nonsmoking patients. Safety and effectiveness of Adempas doses higher than 2.5 mg three times a day have not been established. A dose reduction should be considered in patients who stop smoking.
Strong CYP and P-gp/BCRP inhibitors: Concomitant use of riociguat with strong cytochrome CYP inhibitors and P-gp/BCRP inhibitors such as azole antimycotics (for example, ketoconazole, itraconazole) or HIV protease inhibitors (such as ritonavir) increase riociguat exposure and may result in hypotension. Consider a starting dose of 0.5 mg 3 times a day when initiating Adempas in patients receiving strong CYP and P-gp/BCRP inhibitors. Monitor for signs and symptoms of hypotension on initiation and on treatment with strong CYP and P-gp/BCRP inhibitors. A dose reduction should be considered in patients who may not tolerate the hypotensive effect of riociguat.
Strong CYP3A inducers: Strong inducers of CYP3A (for example, rifampin, phenytoin, carbamazepine, phenobarbital or St. John’s Wort) may significantly reduce riociguat exposure. Data are not available to guide dosing of riociguat when strong CYP3A inducers are co-administered.
Antacids: Antacids such as aluminum hydroxide/magnesium hydroxide decrease riociguat absorption and should not be taken within 1 hour of taking Adempas.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category X
Risk Summary
- Adempas may cause fetal harm when administered to a pregnant woman and is contraindicated during pregnancy. Adempas was teratogenic and embryotoxic in rats at doses with exposures to unbound drug that were approximately 8 times and 2 times, respectively, the human exposure. In rabbits, riociguat led to abortions at 4 times the human exposure and fetal toxicity with exposures approximately 13 times the human exposure. If Adempas is used in pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to the fetus.
Animal Data
- In rats administered riociguat orally (1, 5, and 25 mg/kg/day) throughout organogenesis, an increased rate of cardiac ventricular-septal defect was observed at the highest dose tested. The highest dose produced evidence of maternal toxicity (reduced body weight). Post-implantation loss was statistically significantly increased from the mid-dose of 5 mg/kg/day. Plasma exposure at the lowest dose in which no adverse effects were observed is approximately 0.4 times that in humans at the maximally recommended human dose (MRHD) of 2.5 mg three times a day based on area under the time-concentration curve (AUC) for unbound drug in rat and humans. Plasma exposure at the highest dose (25 mg/kg/day) is approximately 8 times that in humans at the MRHD while exposure at the mid-dose (5 mg/kg/day) is approximately 2 times that in humans at the MRHD. In rabbits given doses of 0.5, 1.5 and 5 mg/kg/day, an increase in spontaneous abortions was observed starting at the middle dose of 1.5 mg/kg, and an increase in resorptions was observed at 5 mg/kg/day. Plasma exposures at these doses were 4 times and 13 times, respectively, the human exposure at the MRHD.
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Riociguat in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Riociguat during labor and delivery.
Nursing Mothers
- It is not known if Adempas is present in human milk. Riociguat or its metabolites were present in the milk of rats. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from riociguat, discontinue nursing or Adempas.
Pediatric Use
- Safety and effectiveness of Adempas in pediatric patients have not been established.
Geriatic Use
- Of the total number of subjects in clinical studies of Adempas, 23% were 65 and over, and 6% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
- Elderly patients showed a higher exposure to Adempas.
Gender
There is no FDA guidance on the use of Riociguat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Riociguat with respect to specific racial populations.
Renal Impairment
- Safety and efficacy have not been demonstrated in patients with creatinine clearance <15 mL/min or on dialysis.
Hepatic Impairment
- Safety and efficacy have not been demonstrated in patients with severe hepatic impairment (Child Pugh C)
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Riociguat in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Riociguat in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Riociguat in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Riociguat in the drug label.
Overdosage
- In cases of overdose, blood pressure should be closely monitored and supported as appropriate. Based on extensive plasma protein binding, riociguat is not expected to be dialyzable.
Pharmacology
There is limited information regarding Riociguat Pharmacology in the drug label.
Mechanism of Action
- Riociguat is a stimulator of soluble guanylate cyclase (sGC), an enzyme in the cardiopulmonary system and the receptor for nitric oxide (NO).
- When NO binds to sGC, the enzyme catalyzes synthesis of the signaling molecule cyclic guanosine monophosphate (cGMP). Intracellular cGMP plays an important role in regulating processes that influence vascular tone, proliferation, fibrosis and inflammation.
- Pulmonary hypertension is associated with endothelial dysfunction, impaired synthesis of nitric oxide and insufficient stimulation of the NO-sGC-cGMP pathway.
- Riociguat has a dual mode of action. It sensitizes sGC to endogenous NO by stabilizing the NO-sGC binding. Riociguat also directly stimulates sGC via a different binding site, independently of NO.
- Riociguat stimulates the NO-sGC-cGMP pathway and leads to increased generation of cGMP with subsequent vasodilation.
- The active metabolite (M1) of riociguat is 1/3 to 1/10 as potent as riociguat.
Structure
- Adempas (riociguat) is a tablet for oral administration. Riociguat is methyl 4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo [3,4-b]pyridin-3-yl]-5-pyrimidinyl(methyl)carbamate with the following structural formula:

- Riociguat is a white to yellowish, crystalline, non-hygroscopic substance with a molecular weight of 422.42 g/mol. In solid form it is stable to temperature, light, and humidity.
- The solubility at 25°C in water: 4 mg/L, in ethanol: 800 mg/L, in 0.1 HCl (pH 1): 250 mg/L and in buffer (phosphate) pH 7: 3 mg/L. In the pH range of 2 to 4 the solubility showed strong pH-dependency. Solubility increases at lower pH values.
- Each round film-coated tablet contains 0.5 mg (1.0, 1.5, 2.0, 2.5 mg) riociguat. The inactive ingredients are cellulose microcrystalline, crospovidone, hypromellose 5cP, lactose monohydrate, magnesium stearate, sodium laurylsulfate, hydroxypropylcellulose, hypromellose 3cP, propylene glycol, and titanium dioxide. Adempas 1, 1.5, 2 and 2.5 mg tablets contain, in addition, ferric oxide yellow. Adempas 2 and 2.5 mg tablets contain, in addition, ferric oxide red..
Pharmacodynamics
- There is a direct relationship between riociguat plasma concentration and hemodynamic parameters such as systemic vascular resistance, systolic blood pressure, pulmonary vascular resistance (PVR), and cardiac output.
- Hemodynamic parameters were assessed in CTEPH patients in CHEST-1. Right heart catheterization was performed at the beginning and the end of the study period in 233 patients. A statistically significant reduction of PVR (-246 dyn*s*cm-5) was shown in the Adempas group vs. placebo. Improvements in other hemodynamic parameters (not pre-specified as endpoints) are displayed in Table 2 below.

- Hemodynamic parameters were assessed in PAH patients in PATENT-1. Right heart catheterization was performed at the beginning and the end of the study period in 339 patients.
- A statistically significant reduction of PVR (-226 dyn*sec*cm-5) was shown in the Adempas individual titration group (to maximum dose of 2.5 mg three times a day) vs. placebo. Improvement in other relevant hemodynamic parameters (not pre-specified as endpoints) for the individual dose titration group versus placebo are displayed in Table 3.

Biomarkers
- In the CHEST-1 study, Adempas significantly reduced N-terminal prohormone of brain natriuretic peptide (NT-proBNP), placebo-corrected mean change from baseline -444 ng/L, 95% CI -843 to -45. In the PATENT-1 study Adempas demonstrated a statistically significant reduction of NT-proBNP, placebo‑corrected mean change from baseline: -432 ng/L, 95% CI –782 to –82.
Pharmacodynamic interactions
Nitrates: Riociguat 2.5 mg tablets potentiated the blood pressure lowering effect of sublingual nitroglycerin (0.4 mg) taken 4 and 8 hours after riociguat. Syncope was reported in some patients [see Contraindications (4.2)].
Phosphodiesterase-5 inhibitors: In an exploratory interaction study in 7 patients with PAH on stable sildenafil treatment (20 mg three times a day), single doses of riociguat (0.5 mg and 1 mg sequentially) showed additive hemodynamic effects.
Among patients with PAH on stable sildenafil treatment (20 mg, three times a day) and riociguat (1 to 2.5 mg, three times a day) there was one death, possibly related to the combination of these drugs, and a high rate of discontinuation for hypotension.
Warfarin: Concomitant administration of riociguat and warfarin did not alter prothrombin time.
Acetylsalicylic Acid: Concomitant use of riociguat and aspirin did not affect bleeding time or platelet aggregation
Pharmacokinetics
- Riociguat pharmacokinetics are dose proportional from 0.5 to 2.5 mg. Inter-individual variability of riociguat exposure (AUC) across all doses is approximately 60%, and within-subject variability is approximately 30%.
Absorption and distribution
- The absolute bioavailability of riociguat is about 94%. Peak plasma riociguat concentrations were observed within 1.5 hours after tablet intake. Food does not affect the bioavailability of riociguat.
- The volume of distribution at steady state is approximately 30 L. Plasma protein binding in humans is approximately 95%, with serum albumin and α1–acidic glycoprotein being the main binding components.
- Riociguat is a substrate of P-gp and BCRP.
Metabolism and excretion
- Riociguat is mainly cleared by metabolism by CYP1A1, CYP3A, CYP2C8 and CYP2J2. Formation of the major active metabolite, M1, is catalyzed by CYP1A1, which is inducible by polycyclic aromatic hydrocarbons such as those present in cigarette smoke. M1 is further metabolized to the inactive N-glucuronide. Plasma concentrations of M1 in patients with PAH are about half those for riociguat.
- Following oral administration of radiolabeled riociguat in healthy individuals, about 40 and 53% of the total radioactivity was recovered in urine and feces, respectively. There appears to be considerable variability in the proportion of metabolites and unchanged riociguat excreted, but metabolites were the major components of the dose excreted in most individuals.
- Average systemic clearance of riociguat was about 1.8 L/h in patients with PAH and about 3.4 L/h in healthy subjects. The terminal elimination half-life is about 12 hours in patients and 7 hours in healthy subjects.
Specific Populations: The effect of intrinsic factors on riociguat and M1 are shown below in Figure 1. There are no clinically relevant effects of age, sex, weight, or race/ethnicity on the pharmacokinetics of riociguat or M1. No dose adjustment is warranted.

Drug interactions: The effect of other extrinsic factors on riociguat and M1 were studied in healthy subjects and are shown in Figure 2
- HIV protease inhibitors are strong CYP3A inhibitors and may increase riociguat plasma concentrations to levels similar to those seen with ketoconazole. ** AUC only, estimated using population pharmacokinetics methods *** AUC only for metabolite, estimated using population pharmacokinetics methods. **** Monitor for signs and symptoms of hypotension on initiation and on treatment with strong CYP and P-gp/BCRP inhibitors.
Strong CYP3A inducers: Data are not available to inform dosing of riociguat when strong CYP3A inducers are co-administered.
Effects of Riociguat on other Drugs: Riociguat did not affect the pharmacokinetics of midazolam, warfarin, or sildenafil.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Riociguat in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Riociguat in the drug label.
How Supplied
Storage
There is limited information regarding Riociguat Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Riociguat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Riociguat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Riociguat in the drug label.
Precautions with Alcohol
- Alcohol-Riociguat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Riociguat
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Riociguat |Label Name=Riociguat11.png
}}
{{#subobject:
|Label Page=Riociguat |Label Name=Riociguat11.png
}}