Ribavirin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
RISK OF SERIOUS DISORDERS AND RIBAVIRIN-ASSOCIATED EFFECTS
See full prescribing information for complete Boxed Warning.
Ribavirin monotherapy is not effective for the treatment of chronic hepatitis C virus infection and should not be used alone for this indication. The primary clinical toxicity of ribavirin is hemolytic anemia. The anemia associated with ribavirin therapy may result in worsening of cardiac disease and lead to fatal and nonfatal myocardial infarctions. Patients with a history of significant or unstable cardiac disease should not be treated with ribavirin.
Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. In addition, ribavirin has a multiple dose half-life of 12 days, and it may persist in non-plasma compartments for as long as 6 months. Therefore, ribavirin, including ribavirin tablets, is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Extreme care must be taken to avoid pregnancy during therapy and for 6 months after completion of therapy in both female patients and in female partners of male patients who are taking ribavirin therapy. At least two reliable forms of effective contraception must be utilized during treatment and during the 6 month post treatment follow-up period:
|
Overview
Ribavirin is a nucleoside analogue that is FDA approved for the treatment of chronic hepatitis C (CHC) virus infection in combination with peginterferon alfa-2a in adults with compensated liver disease not previously treated with interferon alpha, and in CHC patients coinfected with HIV-1. There is a Black Box Warning for this drug as shown here. Common adverse reactions include injection site reaction, pruritus, weight decrease, diarrhea, gastrointestinal symptoms, loss of appetite, nausea, vomiting, neutropenia, asthenia, dizziness, excluding vertigo, headache, insomnia, fatigue and influenza-like illness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Chronic Hepatitis C Monoinfection
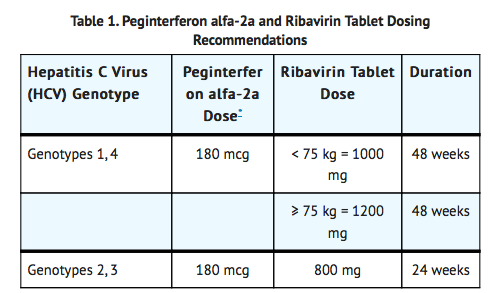
- Dosage: 800-1200 mg/day, administered in two divided doses, for 24-48 week. Administer with food. Exact dose depends on the genotype of the Hepatitis C Virus (HCV) and weight of the patients.
Chronic Hepatitis C with HIV Coinfection
- Dosage: peg-interferon alfa-2a 180 mcg subcutaneous once weekly and ribavirin tablets, 800 mg PO daily for a total duration of 48 weeks, regardless of HCV genotype. Ribavirin tablets should be taken with food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Preemptive Adenovirus Infection in Hematopoietic Cell Transplants
- Ribavirin 15 mg/kg orally three times daily for 4 days, then 8 mg/kg three times daily for up to 10 days[1].
Non–Guideline-Supported Use
Herpes Simplex Infection
- Dosage: 400 mg 4 times a day for 3 days followed by 400 mg twice a day for 5 days[2].
Parainfluenza Virus-3 Infection
- Dosage: 6 g/day in 3 divided doses for 5 to 7 days (Aerosol RIbavirin)[3].
Treatment of Influenza A and B Virus
- Dosage: 1200 as initial dose, repeated at 1 and 2 hours; maintaining doses of 1200 mg were given every 12 hours during 2 days. In total, 8.4g in 2 days[4].
Viral Hemorragic Fever
- Dosage:
- Loading dose: 30 mg/kg IV once (maximum 2 g)
- Maintenance
- First Stage: 6 mg/kg IV (maximum 1 g/dose) every 6 hours for 4 days.
Second Stage: 8 mg/kg IV (maximum 500 mg/dose) every 8 hours for 6 days[5].
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Respiratory Syncytial Virus
- Dosage: The recommended treatment regimen is 20 mg/mL RIbavirin as the starting solution in the drug reservoir of the SPAG-2 unit, with continuous aerosol administration for 12-18 hours per day for 3 to 7 days. Using the recommended drug concentration of 20 mg/mL the average aerosol concentration for a 12 hour delivery period would be 190 micrograms/liter of air. Aerosolized Ribavirin should not be administered in a mixture for combined aerosolization or simultaneously with other aerosolized medications.
Preemptive Respiratory Syncytial Virus Infection in Hematopoietic Cell Transplants
- Dosage: 2 g 3 PO times daily[6].
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Preemptive Adenovirus Infection in Hematopoietic Cell Transplants
- Ribavirin 15 mg/kg orally three times daily for 4 days, then 8 mg/kg three times daily for up to 10 days[7].
Chronic Hepatitis C with HIV Coinfection
Hepatitis C virus treatment is indicated for all children with HIV/HCV co-infection 3 years or older.
- Dosage
- Regardless of HCV Genotype
- Peginterferon-2-alfa 180 micrograms/1.73 m2 (Max 180 micrograms) or (peginterferon-2-beta 60 micrograms/m2 body surface area) subcutaneous once weekly
- Oral ribavirin 7.5 mg/kg twice daily for 48 weeks [8]
- Regardless of HCV Genotype
Non–Guideline-Supported Use
Herpes Simplex Infection
- Dosage: 400 mg 4 times a day for 3 days followed by 400 mg twice a day for 5 days[2].
Laryngeal Papillomatosis
- Dosage: 25 mg/kg/day orally [9].
Contraindications
Tablets
Ribavirin tablets are contraindicated in:
- Women who are pregnant. Ribavirin tablets may cause fetal harm when administered to a pregnant woman. *Ribavirin tablets are contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Men whose female partners are pregnant.
- Patients with hemoglobinopathies (e.g., thalassemia major or sickle-cell anemia).
- In combination with didanosine. Reports of fatal hepatic failure, as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis have been reported in clinical trials.
Ribavirin tablets and peginterferon alfa-2a combination therapy is contraindicated in patients with:
- Autoimmune hepatitis.
- Hepatic decompensation (Child-Pugh score greater than 6; class B and C) in cirrhotic CHC monoinfected patients before treatment
- Hepatic decompensation (Child-Pugh score greater than or equal to 6) in cirrhotic CHC patients coinfected with HIV before treatment
Aerosol
Ribavirin is contraindicated in individuals who have shown hypersensitivity to the drug or its components, and in women who are or may become pregnant during exposure to the drug. Ribavirin has demonstrated significant teratogenic and/or embryocidal potential in all animal species in which adequate studies have been conducted (rodents and rabbits). Therefore, although clinical studies have not been performed, it should be assumed that ribavirin may cause fetal harm in humans. Studies in which the drug has been administered systemically demonstrate that ribavirin is concentrated in the red blood cells and persists for the life of the erythrocyte.
Warnings
|
RISK OF SERIOUS DISORDERS AND RIBAVIRIN-ASSOCIATED EFFECTS
See full prescribing information for complete Boxed Warning.
Ribavirin monotherapy is not effective for the treatment of chronic hepatitis C virus infection and should not be used alone for this indication. The primary clinical toxicity of ribavirin is hemolytic anemia. The anemia associated with ribavirin therapy may result in worsening of cardiac disease and lead to fatal and nonfatal myocardial infarctions. Patients with a history of significant or unstable cardiac disease should not be treated with ribavirin.
Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. In addition, ribavirin has a multiple dose half-life of 12 days, and it may persist in non-plasma compartments for as long as 6 months. Therefore, ribavirin, including ribavirin tablets, is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Extreme care must be taken to avoid pregnancy during therapy and for 6 months after completion of therapy in both female patients and in female partners of male patients who are taking ribavirin therapy. At least two reliable forms of effective contraception must be utilized during treatment and during the 6 month post treatment follow-up period:
|
Pregnancy
- Ribavirin may cause birth defects and/or death of the exposed fetus. Ribavirin has demonstrated significant teratogenic and/or embryocidal effects in all animal species in which adequate studies have been conducted. These effects occurred at doses as low as one twentieth of the recommended human dose of ribavirin.
- Ribavirin therapy should not be started unless a report of a negative pregnancy test has been obtained immediately prior to planned initiation of therapy. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients. Patients should be instructed to use at least two forms of effective contraception during treatment and for 6 months after treatment has been stopped. Pregnancy testing should occur monthly during ribavirin therapy and for 6 months after therapy has stopped.
Anemia
- The primary toxicity of ribavirin is hemolytic anemia, which was observed in approximately 13% of all ribavirin/peginterferon alfa-2a-treated subjects in clinical trials. Anemia associated with ribavirin occurs within 1 to 2 weeks of initiation of therapy. Because the initial drop in hemoglobin may be significant, it is advised that hemoglobin or hematocrit be obtained pretreatment and at week 2 and week 4 of therapy or more frequently if clinically indicated. Patients should then be followed as clinically appropriate. Caution should be exercised in initiating treatment in any patient with baseline risk of severe anemia (e.g., spherocytosis, history of gastrointestinal bleeding)
- Fatal and nonfatal myocardial infarctions have been reported in patients with anemia caused by ribavirin. Patients should be assessed for underlying cardiac disease before initiation of ribavirin therapy. Patients with preexisting cardiac disease should have electrocardiograms administered before treatment, and should be appropriately monitored during therapy. If there is any deterioration of cardiovascular status, therapy should be suspended or discontinued. Because cardiac disease may be worsened by drug-induced anemia, patients with a history of significant or unstable cardiac disease should not use ribavirin
Hepatic Failure
- Chronic hepatitis C (CHC) patients with cirrhosis may be at risk of hepatic decompensation and death when treated with alpha interferons, including peginterferon alfa-2a. Cirrhotic CHC patients coinfected with HIV receiving highly active antiretroviral therapy (HAART) and interferon alfa-2a with or without ribavirin appear to be at increased risk for the development of hepatic decompensation compared to patients not receiving HAART. In Study NR15961, among 129 CHC/HIV cirrhotic patients receiving HAART, 14 (11%) of these patients across all treatment arms developed hepatic decompensation resulting in 6 deaths. All 14 patients were on NRTIs, including stavudine, didanosine, abacavir, zidovudine, and lamivudine. These small numbers of patients do not permit discrimination between specific NRTIs or the associated risk. During treatment, patients’ clinical status and hepatic function should be closely monitored for signs and symptoms of hepatic decompensation. Treatment with ribavirin/peginterferon alfa-2a should be discontinued immediately in patients with hepatic decompensation.
Hypersensitivity
- Severe acute hypersensitivity reactions (e.g., urticaria, angioedema, bronchoconstriction, and anaphylaxis) have been observed during alpha interferon and ribavirin therapy. If such a reaction occurs, therapy with peginterferon alfa-2a and ribavirin should be discontinued immediately and appropriate medical therapy instituted. Serious skin reactions including vesiculobullous eruptions, reactions in the spectrum of Stevens-Johnson syndrome (erythema multiforme major) with varying degrees of skin and mucosal involvement and exfoliative dermatitis (erythroderma) have been reported in patients receiving peginterferon alfa-2a with and without ribavirin. Patients developing signs or symptoms of severe skin reactions must discontinue therapy.
Renal Impairment
- Ribavirin should not be used in patients with creatinine clearance < 50 mL/min.
Pulmonary Disorders
- Dyspnea, pulmonary infiltrates, pneumonitis, pulmonary hypertension, and pneumonia have been reported during therapy with ribavirin and interferon. Occasional cases of fatal pneumonia have occurred. In addition, sarcoidosis or the exacerbation of sarcoidosis has been reported. If there is evidence of pulmonary infiltrates or pulmonary function impairment, patients should be closely monitored and, if appropriate, combination ribavirin/peginterferon alfa-2a treatment should be discontinued.
Bone Marrow Suppression
- Pancytopenia (marked decreases in RBCs, neutrophils and platelets) and bone marrow suppression have been reported in the literature to occur within 3 to 7 weeks after the concomitant administration of pegylated interferon/ribavirin and azathioprine. In this limited number of patients (n = 8), myelotoxicity was reversible within 4 to 6 weeks upon withdrawal of both HCV antiviral therapy and concomitant azathioprine and did not recur upon reintroduction of either treatment alone. Peginterferon alfa-2a, ribavirin, and azathioprine should be discontinued for pancytopenia, and pegylated interferon/ribavirin should not be re-introduced with concomitant azathioprine.
Pancreatitis
- Ribavirin and peginterferon alfa-2a therapy should be suspended in patients with signs and symptoms of pancreatitis, and discontinued in patients with confirmed pancreatitis.
Laboratory Tests
- Before beginning peginterferon alfa-2a/ribavirin combination therapy, standard hematological and biochemical laboratory tests are recommended for all patients. Pregnancy screening for women of childbearing potential must be performed. Patients who have preexisting cardiac abnormalities should have electrocardiograms administered before treatment with peginterferon alfa-2a/ribavirin.
- After initiation of therapy, hematological tests should be performed at 2 weeks and 4 weeks and biochemical tests should be performed at 4 weeks. Additional testing should be performed periodically during therapy. In the clinical studies, the CBC (including hemoglobin level and white blood cell and platelet counts) and chemistries (including liver function tests and uric acid) were measured at 1, 2, 4, 6, and 8 weeks, and then every 4 to 6 weeks or more frequently if abnormalities were found. Thyroid stimulating hormone (TSH) was measured every 12 weeks. Monthly pregnancy testing should be performed during combination therapy and for 6 months after discontinuing therapy.
The entrance criteria used for the clinical studies of ribavirin and peginterferon alfa-2a may be considered as a guideline to acceptable baseline values for initiation of treatment:
- Platelet count ≥ 90,000 cells/mm3 (as low as 75,000 cells/mm3 in HCV patients with cirrhosis or 70,000 cells/mm3 in patients with CHC and HIV)
- Absolute neutrophil count (ANC) ≥ 1500 cells/mm3
- TSH and T4 within normal limits or adequately controlled thyroid function
- CD4+ cell count ≥ 200 cells/μL or CD4+ cell count ≥ 100 cells/μL but < 200 cells/μL and HIV-1 RNA < 5000 copies/mL in patients coinfected with HIV
- Hemoglobin ≥ 12 g/dL for women and ≥ 13 g/dL for men in CHC monoinfected patients
- Hemoglobin ≥ 11 g/dL for women and ≥ 12 g/dL for men in patients with CHC and HIV
Adverse Reactions
Clinical Trials Experience
Application Site Disorder
- Injection Site Reaction
Endocrine Disorders
Flu-Like Symptoms
Gastrointestinal Effects
Hematologic Effects
Metabolic and Nutritional
Musculoeskeletal, Connective Tissue and Bone
Neurological Effects
- Headache
- Dizziness (excluding vertigo)
- Memory impairment
Psychiatric Effects
Respiratory, Thoracic and Mediastinal
Skin and Subcutaneous Tissue
Visual Disorders
Postmarketing Experience
Blood and Lymphatic System disorders
- Pure red cell aplasia
Ear and Labyrinth disorders
Eye disorders
Immune disorders
- Renal graft rejection
- Liver graft rejection
Metabolism and Nutrition disorders
Skin and Subcutaneous Tissue disorders
Drug Interactions
Results from a pharmacokinetic sub-study demonstrated no pharmacokinetic interaction between peginterferon alfa-2a and ribavirin.
Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
In vitro data indicate ribavirin reduces phosphorylation of lamivudine, stavudine, and zidovudine. However, no pharmacokinetic (e.g., plasma concentrations or intracellular triphosphorylated active metabolite concentrations) or pharmacodynamic (e.g., loss of HIV/HCV virologic suppression) interaction was observed when ribavirin and lamivudine (n = 18), stavudine (n = 10), or zidovudine (n = 6) were coadministered as part of a multi-drug regimen to HCV/HIV coinfected patients. In Study NR15961 among the CHC/HIV coinfected cirrhotic patients receiving NRTIs cases of hepatic decompensation (some fatal) were observed.
Patients receiving peginterferon alfa-2a/ribavirin and NRTIs should be closely monitored for treatment associated toxicities. Physicians should refer to prescribing information for the respective NRTIs for guidance regarding toxicity management. In addition, dose reduction or discontinuation of peginterferon alfa-2a, ribavirin or both should also be considered if worsening toxicities are observed, including hepatic decompensation (e.g., Child-Pugh ≥ 6).
Didanosine
Coadministration of ribavirin and didanosine is contraindicated. Didanosine or its active metabolite (dideoxyadenosine 5’-triphosphate) concentrations are increased when didanosine is coadministered with ribavirin, which could cause or worsen clinical toxicities. Reports of fatal hepatic failure, as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis have been reported in clinical trials.
Zidovudine
In Study NR15961, patients who were administered zidovudine in combination with peginterferon alfa-2a/ribavirin developed severe neutropenia (ANC < 500) and severe anemia (hemoglobin < 8 g/dL) more frequently than similar patients not receiving zidovudine (neutropenia 15% vs. 9%) (anemia 5% vs. 1%). Discontinuation of zidovudine should be considered as medically appropriate.
Drugs Metabolized by Cytochrome P450
In vitro studies indicate that ribavirin does not inhibit CYP 2C9, CYP 2C19, CYP 2D6 or CYP 3A4.
Azathioprine
The use of ribavirin to treat chronic hepatitis C in patients receiving azathioprine has been reported to induce severe pancytopenia and may increase the risk of azathioprine-related myelotoxicity. Inosine monophosphate dehydrogenase (IMDH) is required for one of the metabolic pathways of azathioprine. Ribavirin is known to inhibit IMDH, thereby leading to accumulation of an azathioprine metabolite, 6-methylthioinosine monophosphate (6-MTITP), which is associated with myelotoxicity (neutropenia, thrombocytopenia, and anemia). Patients receiving azathioprine with ribavirin should have complete blood counts, including platelet counts, monitored weekly for the first month, twice monthly for the second and third months of treatment, then monthly or more frequently if dosage or other therapy changes are necessary.
Use in Specific Populations
Pregnancy
Teratogenic Effects
- Ribavirin produced significant embryocidal and/or teratogenic effects in all animal species in which adequate studies have been conducted. Malformations of the skull, palate, eye, jaw, limbs, skeleton, and gastrointestinal tract were noted. The incidence and severity of teratogenic effects increased with escalation of the drug dose. Survival of fetuses and offspring was reduced.
In conventional embryotoxicity/teratogenicity studies in rats and rabbits, observed no-effect dose levels were well below those for proposed clinical use (0.3 mg/kg/day for both the rat and rabbit; approximately 0.06 times the recommended daily human dose of ribavirin). No maternal toxicity or effects on offspring were observed in a peri/postnatal toxicity study in rats dosed orally at up to 1 mg/kg/day (approximately 0.01 times the maximum recommended daily human dose of ribavirin).
Treatment and Post treatment: Potential Risk to the Fetus
Ribavirin is known to accumulate in intracellular components from where it is cleared very slowly. It is not known whether ribavirin is contained in sperm, and if so, will exert a potential teratogenic effect upon fertilization of the ova. However, because of the potential human teratogenic effects of ribavirin, male patients should be advised to take every precaution to avoid risk of pregnancy for their female partners.
Ribavirin should not be used by pregnant women or by men whose female partners are pregnant. Female patients of childbearing potential and male patients with female partners of childbearing potential should not receive ribavirin unless the patient and his/her partner are using effective contraception (two reliable forms) during therapy and for 6 months post therapy.
Ribavirin Pregnancy Registry
A Ribavirin Pregnancy Registry has been established to monitor maternal-fetal outcomes of pregnancies of female patients and female partners of male patients exposed to ribavirin during treatment and for 6 months following cessation of treatment. Healthcare providers and patients are encouraged to report such cases by calling 1-800-593-2214
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ribavirin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ribavirin during labor and delivery.
Nursing Mothers
It is not known whether ribavirin is excreted in human milk. Because many drugs are excreted in human milk and to avoid any potential for serious adverse reactions in nursing infants from ribavirin, a decision should be made either to discontinue nursing or therapy with ribavirin, based on the importance of the therapy to the mother.
Pediatric Use
- Pharmacokinetic evaluations in pediatric patients have not been performed.
- Safety and effectiveness of ribavirin have not been established in patients below the age of 18.
Geriatic Use
Clinical studies of ribavirin and peginterferon alfa-2a did not include sufficient numbers of subjects aged 65 or over to determine whether they respond differently from younger subjects. Specific pharmacokinetic evaluations for ribavirin in the elderly have not been performed. The risk of toxic reactions to this drug may be greater in patients with impaired renal function. Ribavirin should not be administered to patients with creatinine clearance < 50 mL/min.
Gender
No clinically significant differences in the pharmacokinetics of ribavirin were observed between male and female subjects.
Ribavirin pharmacokinetics, when corrected for weight, are similar in male and female patients.
Race
A pharmacokinetic study in 42 subjects demonstrated there is no clinically significant difference in ribavirin pharmacokinetics among Black (n = 14), Hispanic (n = 13) and Caucasian (n = 15) subjects.
Renal Impairment
The pharmacokinetics of ribavirin following administration of ribavirin have not been studied in patients with renal impairment and there are limited data from clinical trials on administration of ribavirin in patients with creatinine clearance < 50 mL/min. Therefore, patients with creatinine clearance < 50 mL/min should not be treated with ribavirin.
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of ribavirin following administration of ribavirin has not been evaluated. The clinical trials of ribavirin were restricted to patients with Child-Pugh class A disease.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ribavirin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ribavirin in patients who are immunocompromised.
Organ Transplant Recipients
The safety and efficacy of peginterferon alfa-2a and ribavirin treatment have not been established in patients with liver and other transplantations. As with other alpha interferons, liver and renal graft rejections have been reported on peginterferon alfa-2a, alone or in combination with ribavirin
Administration and Monitoring
Administration
There is limited information regarding Ribavirin Administration in the drug label.
Monitoring
Before beginning peginterferon alfa-2a/ribavirin combination therapy, standard hematological and biochemical laboratory tests are recommended for all patients. Pregnancy screening for women of childbearing potential must be performed. Patients who have preexisting cardiac abnormalities should have electrocardiograms administered before treatment with peginterferon alfa-2a/ribavirin.
After initiation of therapy, hematological tests should be performed at 2 weeks and 4 weeks and biochemical tests should be performed at 4 weeks. Additional testing should be performed periodically during therapy. In the clinical studies, the CBC (including hemoglobin level and white blood cell and platelet counts) and chemistries (including liver function tests and uric acid) were measured at 1, 2, 4, 6, and 8 weeks, and then every 4 to 6 weeks or more frequently if abnormalities were found. Thyroid stimulating hormone (TSH) was measured every 12 weeks. Monthly pregnancy testing should be performed during combination therapy and for 6 months after discontinuing therapy.
The entrance criteria used for the clinical studies of ribavirin and peginterferon alfa-2a may be considered as a guideline to acceptable baseline values for initiation of treatment:
- Platelet count ≥ 90,000 cells/mm3 (as low as 75,000 cells/mm3 in HCV patients with cirrhosis or 70,000 cells/mm3 in patients with CHC and HIV)
- Absolute neutrophil count (ANC) ≥ 1500 cells/mm3
- TSH and T4 within normal limits or adequately controlled thyroid function
- CD4+ cell count ≥ 200 cells/μL or CD4+ cell count ≥ 100 cells/μL but < 200 cells/μL and HIV-1 RNA < 5000 copies/mL in patients coinfected with HIV
- Hemoglobin ≥ 12 g/dL for women and ≥ 13 g/dL for men in CHC monoinfected patients
- Hemoglobin ≥ 11 g/dL for women and ≥ 12 g/dL for men in patients with CHC and HIV
IV Compatibility
There is limited information regarding the compatibility of Ribavirin and IV administrations.
Overdosage
No cases of overdose with ribavirin have been reported in clinical trials. Hypocalcemia and hypomagnesemia have been observed in persons administered greater than the recommended dosage of ribavirin. In most of these cases, ribavirin was administered intravenously at dosages up to and in some cases exceeding four times the recommended maximum oral daily dose.
Pharmacology
Mechanism of Action
There is limited information regarding Ribavirin Mechanism of Action in the drug label.
Structure
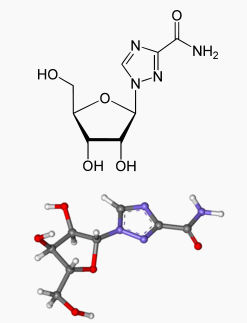
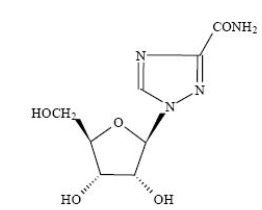
Ribavirin is a nucleoside analogue with antiviral activity. The chemical name of ribavirin is 1-β-D-ribofuranosyl-1 H-1,2,4-triazole-3-carboxamide and has the following structural formula:
Pharmacodynamics
There is limited information regarding Ribavirin Pharmacodynamics in the drug label.
Pharmacokinetics
Multiple dose ribavirin pharmacokinetic data are available for HCV patients who received ribavirin in combination with peginterferon alfa-2a. Following administration of 1200 mg/day with food for 12 weeks mean ± SD (n = 39; body weight > 75 kg) AUC0-12hr was 25,361 ± 7110 ng•hr/mL and Cmax was 2748 ± 818 ng/mL. The average time to reach Cmax was 2 hours. Trough ribavirin plasma concentrations following 12 weeks of dosing with food were 1662 ± 545 ng/mL in HCV infected patients who received 800 mg/day (n = 89), and 2112 ± 810 ng/mL in patients who received 1200 mg/day (n = 75; body weight > 75 kg).
The terminal half-life of ribavirin following administration of a single oral dose of ribavirin is about 120 to 170 hours. The total apparent clearance following administration of a single oral dose of ribavirin is about 26 L/h. There is extensive accumulation of ribavirin after multiple dosing (twice daily) such that the Cmax at steady state was four-fold higher than that of a single dose.
Effect of Food on Absorption of Ribavirin
Bioavailability of a single oral dose of ribavirin was increased by coadministration with a high-fat meal. The absorption was slowed (Tmax was doubled) and the AUC0-192h and Cmax increased by 42% and 66%, respectively, when ribavirin was taken with a high-fat meal compared with fasting conditions [see Dosage and Administration (2.1) and Patient Counseling Information (17)].
Elimination and Metabolism
The contribution of renal and hepatic pathways to ribavirin elimination after administration of ribavirin is not known. In vitro studies indicate that ribavirin is not a substrate of CYP450 enzymes.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a p53 (+/-) mouse carcinogenicity study up to the maximum tolerated dose of 100 mg/kg/day, ribavirin was not oncogenic. Ribavirin was also not oncogenic in a rat 2 year carcinogenicity study at doses up to the maximum tolerated dose of 60 mg/kg/day. On a body surface area basis, these doses are approximately 0.5 and 0.6 times the maximum recommended daily human dose of ribavirin, respectively.
Mutagenesis
Ribavirin demonstrated mutagenic activity in the in vitro mouse lymphoma assay. No clastogenic activity was observed in an in vivo mouse micronucleus assay at doses up to 2000 mg/kg. However, results from studies published in the literature show clastogenic activity in the in vivo mouse micronucleus assay at oral doses up to 2000 mg/kg. A dominant lethal assay in rats was negative, indicating that if mutations occurred in rats they were not transmitted through male gametes. However, potential carcinogenic risk to humans cannot be excluded.
Impairment of Fertility
In a fertility study in rats, ribavirin showed a marginal reduction in sperm counts at the dose of 100 mg/kg/day with no effect on fertility. Upon cessation of treatment, total recovery occurred after 1 spermatogenesis cycle. Abnormalities in sperm were observed in studies in mice designed to evaluate the time course and reversibility of ribavirin-induced testicular degeneration at doses of 15 to 150 mg/kg/day (approximately 0.1 to 0.8 times the maximum recommended daily human dose of ribavirin) administered for 3 to 6 months. Upon cessation of treatment, essentially total recovery from ribavirin-induced testicular toxicity was apparent within 1 or 2 spermatogenic cycles.
Female patients of childbearing potential and male patients with female partners of childbearing potential should not receive ribavirin unless the patient and his/her partner are using effective contraception (two reliable forms). Based on a multiple dose half-life (t1/2) of ribavirin of 12 days, effective contraception must be utilized for 6 months post therapy (i.e., 15 half-lives of clearance for ribavirin).
No reproductive toxicology studies have been performed using peginterferon alfa-2a in combination with ribavirin. However, peginterferon alfa-2a and ribavirin when administered separately, each has adverse effects on reproduction. It should be assumed that the effects produced by either agent alone would also be caused by the combination of the two agents.
Animal Toxicology and/or Pharmacology
In a study in rats, it was concluded that dominant lethality was not induced by ribavirin at doses up to 200 mg/kg for 5 days (up to 1.7 times the maximum recommended human dose of ribavirin).
Long-term studies in the mouse and rat (18 to 24 months; dose 20 to 75, and 10 to 40 mg/kg/day, respectively, approximately 0.1 to 0.4 times the maximum daily human dose of ribavirin) have demonstrated a relationship between chronic ribavirin exposure and an increased incidence of vascular lesions (microscopic hemorrhages) in mice. In rats, retinal degeneration occurred in controls, but the incidence was increased in ribavirin-treated rats.
Clinical Studies
Chronic Hepatitis C Patients
- The safety and effectiveness of peginterferon alfa-2a in combination with ribavirin for the treatment of hepatitis C virus infection were assessed in two randomized controlled clinical trials. All patients were adults, had compensated liver disease, detectable hepatitis C virus, liver biopsy diagnosis of chronic hepatitis, and were previously untreated with interferon. Approximately 20% of patients in both studies had compensated cirrhosis (Child-Pugh class A). Patients coinfected with HIV were excluded from these studies.
- In Study NV15801, patients were randomized to receive either peginterferon alfa-2a 180 mcg subcutaneous once weekly with an oral placebo, peginterferon alfa-2a 180 mcg once weekly with ribavirin 1000 mg by mouth (body weight < 75 kg) or 1200 mg by mouth (body weight ≥ 75 kg) or interferon alfa-2b 3 MIU subcutaneous three times a week plus ribavirin 1000 mg or 1200 mg by mouth. All patients received 48 weeks of therapy followed by 24 weeks of treatment-free follow-up. Ribavirin or placebo treatment assignment was blinded. Sustained virological response was defined as undetectable (< 50 IU/mL) HCV RNA on or after study week 68. Peginterferon alfa-2a in combination with ribavirin resulted in a higher SVR compared to peginterferon alfa-2a alone or interferon alfa-2b and ribavirin (Table 5). In all treatment arms, patients with viral genotype 1, regardless of viral load, had a lower response rate to peginterferon alfa-2a in combination with ribavirin compared to patients with other viral genotypes.
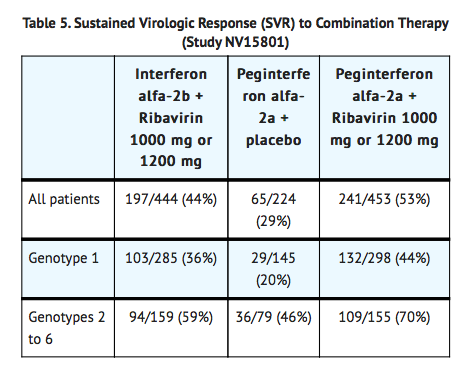
- Difference in overall treatment response (peginterferon alfa-2a/ribavirin – Interferon alfa-2b/ribavirin) was 9% (95% CI 2.3, 15.3). In Study NV15942, all patients received peginterferon alfa-2a 180 mcg subcutaneous once weekly and were randomized to treatment for either 24 or 48 weeks and to a ribavirin dose of either 800 mg or 1000 mg/1200 mg (for body weight < 75 kg/≥ 75 kg). Assignment to the four treatment arms was stratified by viral genotype and baseline HCV viral titer. Patients with genotype 1 and high viral titer (defined as > 2 x 106 HCV RNA copies/mL serum) were preferentially assigned to treatment for 48 weeks.
Sustained Virologic Response and HCV Genotype
- HCV 1 and 4- Irrespective of baseline viral titer, treatment for 48 weeks with peginterferon alfa-2a and 1000 mg or 1200 mg of ribavirin resulted in higher SVR (defined as undetectable HCV RNA at the end of the 24 week treatment-free follow-up period) compared to shorter treatment (24 weeks) and/or 800 mg ribavirin.
- HCV 2 and 3- Irrespective of baseline viral titer, treatment for 24 weeks with peginterferon alfa-2a and 800 mg of ribavirin resulted in a similar SVR compared to longer treatment (48 weeks) and/or 1000 mg or 1200 mg of ribavirin (see TABLE 6).
- The numbers of patients with genotype 5 and 6 were too few to allow for meaningful assessment.

Treatment Response Predictors
- Treatment response rates are lower in patients with poor prognostic factors receiving pegylated interferon alpha therapy. In studies NV15801 and NV15942, treatment response rates were lower in patients older than 40 years (50% vs. 66%), in patients with cirrhosis (47% vs. 59%), in patients weighing over 85 kg (49% vs. 60%), and in patients with genotype 1 with high vs. low viral load (43% vs. 56%). African-American patients had lower response rates compared to Caucasians.
- In studies NV15801 and NV15942, lack of early virologic response by 12 weeks (defined as HCV RNA undetectable or > 2 log10 lower than baseline) was grounds for discontinuation of treatment. Of patients who lacked an early viral response by 12 weeks and completed a recommended course of therapy despite a protocol-defined option to discontinue therapy, 5/39 (13%) achieved an SVR. Of patients who lacked an early viral response by 24 weeks, 19 completed a full course of therapy and none achieved an SVR
Chronic Hepatitis C/HIV Coinfected Patients
- In Study NR15961, patients with CHC/HIV were randomized to receive either peginterferon alfa-2a 180 mcg subcutaneous once weekly plus an oral placebo, peginterferon alfa-2a 180 mcg once weekly plus ribavirin 800 mg by mouth daily or interferon alfa-2a, 3 MIU subcutaneous three times a week plus ribavirin 800 mg by mouth daily. All patients received 48 weeks of therapy and sustained virologic response (SVR) was assessed at 24 weeks of treatment-free follow-up. Ribavirin or placebo treatment assignment was blinded in the peginterferon alfa-2a treatment arms. All patients were adults, had compensated liver disease, detectable hepatitis C virus, liver biopsy diagnosis of chronic hepatitis C, and were previously untreated with interferon. Patients also had CD4+ cell count ≥ 200 cells/mcL or CD4+ cell count ≥ 100 cells/mcL but < 200 cells/mcL and HIV-1 RNA < 5000 copies/mL, and stable status of HIV. Approximately 15% of patients in the study had cirrhosis. Results are shown in Table 7.
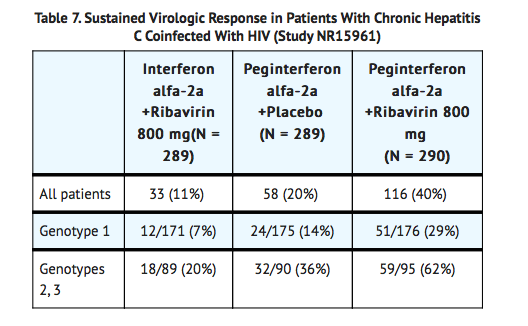
- Treatment response rates were lower in CHC/HIV patients with poor prognostic factors (including HCV genotype 1, HCV RNA > 800,000 IU/mL, and cirrhosis) receiving pegylated interferon alpha therapy.
- Of the patients who did not demonstrate either undetectable HCV RNA or at least a 2 log10 reduction from baseline in HCV RNA titer by 12 weeks of peginterferon alfa-2a and ribavirin combination therapy, 2% (2/85) achieved an SVR.
- In CHC patients with HIV coinfection who received 48 weeks of peginterferon alfa-2a alone or in combination with ribavirin treatment, mean and median HIV RNA titers did not increase above baseline during treatment or 24 weeks post treatment.
How Supplied
Tablets
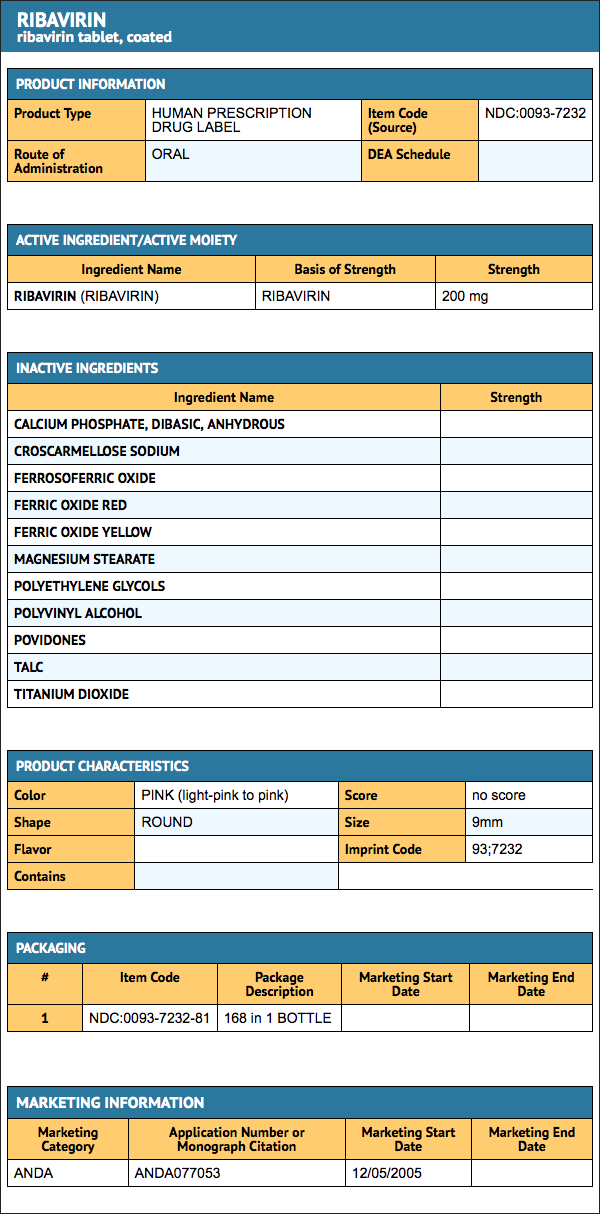
Ribavirin tablets, 200 mg for oral administration are available as follows: Each tablet contains 200 mg of ribavirin and is light-pink to pink, round standard normal convex, coated tablet, debossed with “93” on one side and “7232” on the other side in bottles of 168.
Aerosol
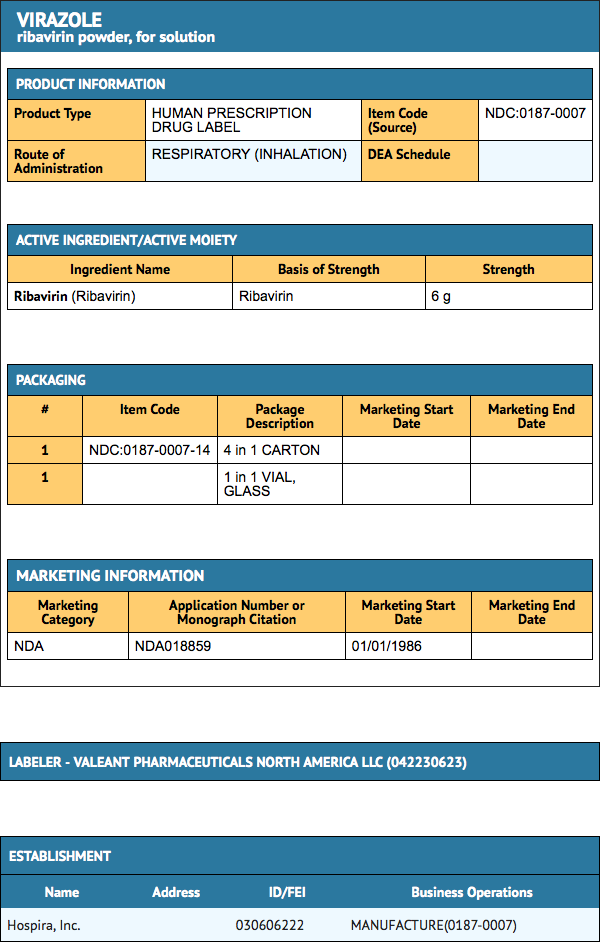
Ribavirin for Inhalation Solution is supplied in four packs containing 100 mL glass vials with 6 grams of Sterile, lyophilized drug (NDC 0187-0007-14) which is to be reconstituted with 300 mL Sterile Water for Injection or Sterile Water for Inhalation (no preservatives added) and administered only by a small particle aerosol generator (SPAG-2)
Storage
Tablet
Store at 20° to 25°C (68° to 77°F). Keep bottle tightly closed. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Aerosol
Vials containing the lyophilized drug powder should be stored in a dry place at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F). Reconstituted solutions may be stored, under sterile conditions, at room temperature (20-30°C, 68-86°F) for 24 hours. Solutions which have been placed in the SPAG-2 unit should be discarded at least every 24 hours.
Images
Drug Images
{{#ask: Page Name::Ribavirin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Tablet

Aerosol
{{#ask: Label Page::Ribavirin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ribavirin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Ribavirin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Ribavirin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Preemptive Adenovirus Infection in Hematopoietic Cell Transplants".
- ↑ 2.0 2.1 Palmieri G, Ambrosi G, Ferraro G, Agrati AM, Palazzini E (1987). "Clinical and immunological evaluation of oral ribavirin administration in recurrent herpes simplex infections". J Int Med Res. 15 (5): 264–75. PMID 3315775.
- ↑ Chakrabarti S, Collingham KE, Holder K, Oyaide S, Pillay D, Milligan DW (2000). "Parainfluenza virus type 3 infections in hematopoetic stem cell transplant recipients: response to ribavirin therapy". Clin Infect Dis. 31 (6): 1516–8. doi:10.1086/317482. PMID 11096028.
- ↑ Stein DS, Creticos CM, Jackson GG, Bernstein JM, Hayden FG, Schiff GM; et al. (1987). "Oral ribavirin treatment of influenza A and B." Antimicrob Agents Chemother. 31 (8): 1285–7. PMC 174922. PMID 3307623.
- ↑ Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB; et al. (2002). "Hemorrhagic fever viruses as biological weapons: medical and public health management". JAMA. 287 (18): 2391–405. PMID 11988060.
- ↑ Boeckh M, Englund J, Li Y, Miller C, Cross A, Fernandez H; et al. (2007). "Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients". Clin Infect Dis. 44 (2): 245–9. doi:10.1086/509930. PMID 17173225.
- ↑ "Preemptive Adenovirus Infection in Hematopoietic Cell Transplants".
- ↑ http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/DB67C4/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/F05BD4/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/evidencexpert.IntermediateToDocumentLink?docId=9258&contentSetId=50. Unknown parameter
|Title=ignored (|title=suggested) (help); Missing or empty|title=(help) - ↑ Balauff A, Sira J, Pearman K, McKiernan P, Buckels J, Kelly D (2001). "Successful ribavirin therapy for life-threatening laryngeal papillomatosis post liver transplantation". Pediatr Transplant. 5 (2): 142–4. PMID 11328555.
- ↑ 10.0 10.1 10.2 10.3 10.4 "PRODUCT INFORMATION REBETOL® (RIBAVIRIN) CAPSULES" (PDF). TGA eBusiness Services. Merck Sharp & Dohme (Australia) Pty Limited. 29 April 2013. Retrieved 23 February 2014.
{{#subobject:
|Page Name=Ribavirin
|Pill Name=
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Ribavirin |Label Name=
}}