Rheumatic fever pathophysiology: Difference between revisions
Varun Kumar (talk | contribs) |
Varun Kumar (talk | contribs) |
||
| Line 33: | Line 33: | ||
Image:Rheumatic fever 004.jpg|Aschoff bodies in rheumatic heart disease | Image:Rheumatic fever 004.jpg|Aschoff bodies in rheumatic heart disease | ||
</gallery> | </gallery> | ||
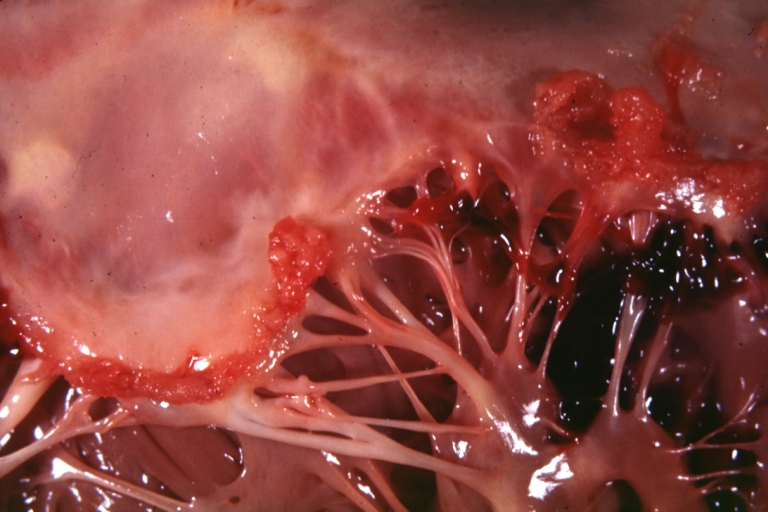
[[Image:ARF mitral valve.jpg|thumb|left|Rheumatic Mitral Valvulitis: Gross; an excellent example of acute rheumatic fever lesion along line of closure of mitral valve <br> <small> [http://www.peir.netImage courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] </small>]] | |||
<br clear="left"/> | |||
==References== | ==References== | ||
Revision as of 21:44, 12 December 2011
|
Rheumatic fever Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rheumatic fever pathophysiology On the Web |
|
American Roentgen Ray Society Images of Rheumatic fever pathophysiology |
|
Risk calculators and risk factors for Rheumatic fever pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Lance Christiansen, D.O.; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Pathophysiology
It was first thought that individuals developed an allergic response to the bacteria, but later it was determined that rheumatic fever was an autoimmunological sequela to a virulent Streptococcus pyogenes infection in a patient who was immunologically sensitized from prior infections by Streptococcus pyogenes. A decrease in frequency and virulence of infections by Streptococcus pyogenes can cause rheumatic fever, to be less frequent and less severe.
Rheumatic fever which depends on a prior autoimmunological stimulation, is a systemic disease affecting the peri-arteriolar connective tissue and can occur after an untreated Group A streptococcal pharyngeal infection. It is believed to be caused by antibody cross-reactivity which is a Type II hypersensitivity reaction and is termed molecular mimicry.
Usually, self reactive B cells remain anergic in the periphery without T cell co-stimulation. During a streptococcal infection activated antigen presenting cells such as macrophages present the bacterial antigen to helper T cells. Helper T cells subsequently activate B cells and induce the production of antibodies against the cell wall of streptococcus. However the antibodies may also act against the myocardium and joints, producing the symptoms of rheumatic fever.
Contrary to the immunological protection developed during most infections, infections by Streptococcus pyogenes cause both a protective immunological and a pathological autoimmunological stimulation and therefore, repeated infections by streptococcus pyogenes will cause both a heightened protective and pathological immune response. The immune response helps in combating the streptococcal infection. However, the autoimmunological response causes an inflammatory, systemic, disease process termed rheumatic fever which is a sequelae to both new streptococcal infection and the prior infections that that triggered the production of autoimmune antibodies.
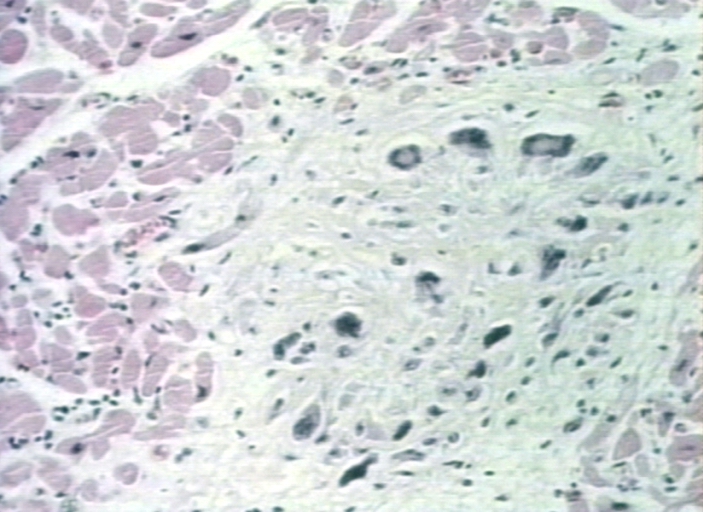
Group A streptococcus pyogenes has a cell wall composed of branched polymers which sometimes contain "M proteins" that are highly antigenic. The antibodies which the immune system generates against the "M proteins" may cross react with cardiac myofiber sarcolemma and smooth muscle cells of arteries, inducing cytokine release and tissue destruction. This inflammation occurs through direct attachment of complement and Fc receptor-mediated recruitment of neutrophils and macrophages. Characteristic Aschoff bodies, characterized by swollen eosinophilic collagen surrounded by lymphocytes and macrophages can be seen on light microscopy. The larger macrophages may become Aschoff giant cells. Acute rheumatic valvular lesions may also involve a cell-mediated immunity reaction as these lesions predominantly contain T-helper cells and macrophages.
In acute rheumatic fever, these lesions may occur in endocardium, myocardium or pericardium. The inflammation may cause a serofibrinous pericardial exudates described as “bread-and-butter” pericarditis, which usually resolves without sequelae. Involvement of the endocardium typically results in fibrinoid necrosis and verrucae formation along the lines of closure of the left heart valves. Warty projections arise from the deposition, while subendothelial lesions may induce irregular thickenings called MacCallum plaques.
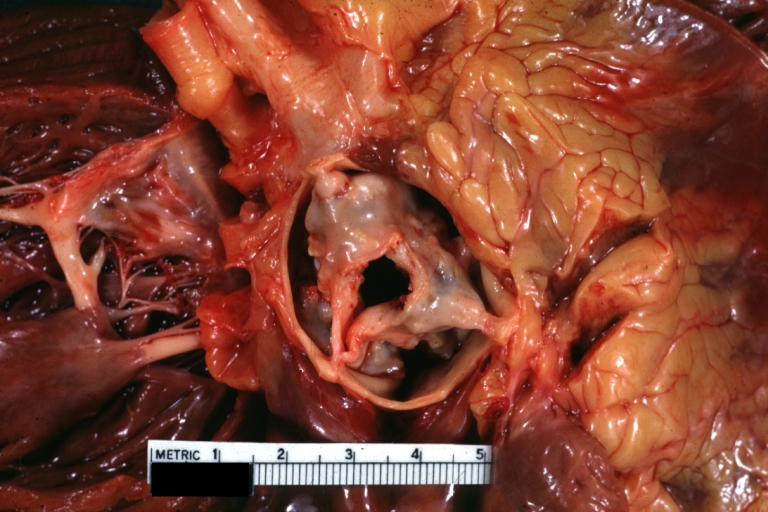
Chronic rheumatic heart disease is characterized by repeated inflammation with fibrinous resolution. The cardinal anatomic changes of the valve include leaflet thickening, commissural fusion and shortening and thickening of the tendinous cords. Rheumatic heart disease cause 99% of mitral stenosis often resulting in a “fish mouth” gross appearance.
Pathological Findings
-
Aortic Stenosis (Tricuspid aorta): Gross, good example of aortic stenosis due to rheumatic fever
-
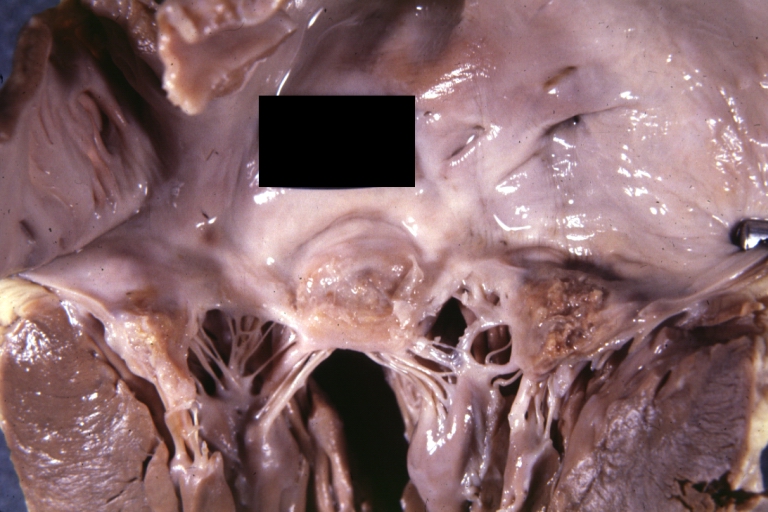
Gross, an excellent example of mitral scarring due to rheumatic fever (healing phase of an infectious lesion).
-
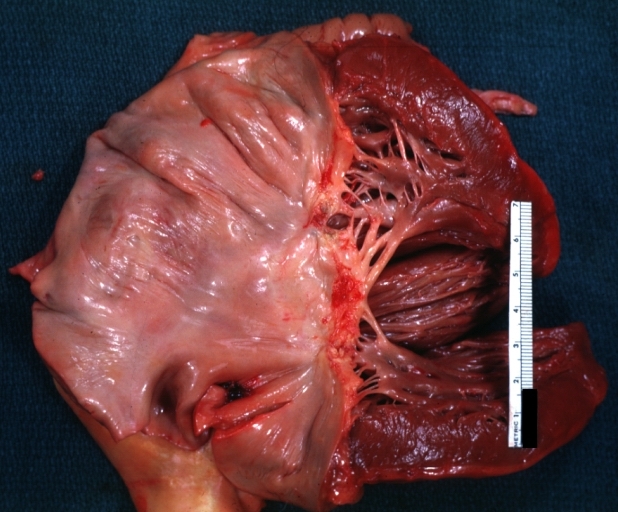
Rheumatic mitral valvulitis: Gross, an excellent example of fibrosis, chorda thickening and shortening has thrombus around the large left atrium
-
Mitral valve: acute rheumatic fever
-
Aschoff bodies in rheumatic heart disease
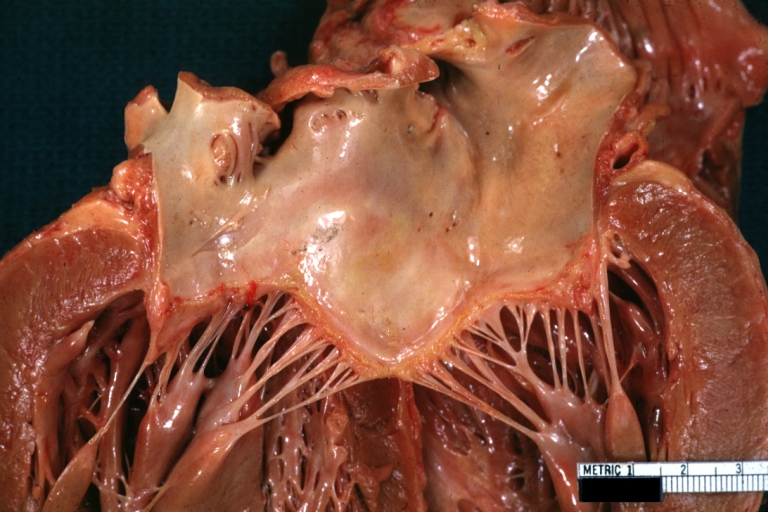
courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology