Repaglinide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 170: | Line 170: | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
* '''Pregnancy Category''' | * '''Pregnancy Category C''' | ||
*Teratogenic Effects | |||
:*Safety in pregnant women has not been established. Repaglinide was not teratogenic in rats or rabbits at doses 40 times (rats) and approximately 0.8 times (rabbit) clinical exposure (on a mg/m2 basis) throughout pregnancy. Because animal reproduction studies are not always predictive of human response, PRANDIN should be used during pregnancy only if it is clearly needed. | |||
:*Because recent information suggests that abnormal blood glucose levels during pregnancy are associated with a higher incidence of congenital abnormalities, many experts recommend that insulin be used during pregnancy to maintain blood glucose levels as close to normal as possible. | |||
*Nonteratogenic Effects | |||
:*Offspring of rat dams exposed to repaglinide at 15 times clinical exposure on a mg/m2 basis during days 17 to 22 of gestation and during lactation developed nonteratogenic skeletal deformities consisting of shortening, thickening, and bending of the humerus during the postnatal period. This effect was not seen at doses up to 2.5 times clinical exposure (on a mg/m2 basis) on days 1 to 22 of pregnancy or at higher doses given during days 1 to 16 of pregnancy. Relevant human exposure has not occurred to date and therefore the safety of PRANDIN administration throughout pregnancy or lactation cannot be established. | |||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
| Line 181: | Line 188: | ||
|useInNursing= | |useInNursing= | ||
*In rat reproduction studies, measurable levels of repaglinide were detected in the breast milk of the dams and lowered blood glucose levels were observed in the pups. Cross fostering studies indicated that skeletal changes (see Nonteratogenic Effects) could be induced in control pups nursed by treated dams, although this occurred to a lesser degree than those pups treated in utero. Although it is not known whether repaglinide is excreted in human milk some oral agents are known to be excreted by this route. Because the potential for hypoglycemia in nursing infants may exist, and because of the effects on nursing animals, a decision should be made as to whether PRANDIN should be discontinued in nursing mothers, or if mothers should discontinue nursing. If PRANDIN is discontinued and if diet alone is inadequate for controlling blood glucose, insulin therapy should be considered. | |||
|useInPed= | |useInPed= | ||
*No studies have been performed in pediatric patients. | |||
|useInGeri= | |useInGeri= | ||
There | |||
*In repaglinide clinical studies of 24 weeks or greater duration, 415 patients were over 65 years of age. In one-year, active-controlled trials, no differences were seen in effectiveness or adverse events between these subjects and those less than 65 other than the expected age-related increase in cardiovascular events observed for PRANDIN and comparator drugs. There was no increase in frequency or severity of hypoglycemia in older subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals to PRANDIN therapy cannot be ruled out. | |||
|useInGender= | |useInGender= | ||
| Line 212: | Line 222: | ||
* Oral | * Oral | ||
|monitoring= | |monitoring= | ||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
| Line 235: | Line 241: | ||
====Signs and Symptoms==== | ====Signs and Symptoms==== | ||
* | *In a clinical trial, patients received increasing doses of PRANDIN up to 80 mg a day for 14 days. There were few adverse effects other than those associated with the intended effect of lowering blood glucose. Hypoglycemia did not occur when meals were given with these high doses. | ||
====Management==== | ====Management==== | ||
* | *Hypoglycemic symptoms without loss of consciousness or neurologic findings should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring may continue until the physician is assured that the patient is out of danger. Patients should be closely monitored for a minimum of 24 to 48 hours, since hypoglycemia may recur after apparent clinical recovery. There is no evidence that repaglinide is dialyzable using hemodialysis. | ||
*Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given a rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of more dilute (10%) glucose solution at a rate that will maintain the blood glucose at a level above 100 mg/dL. | |||
===Chronic Overdose=== | ===Chronic Overdose=== | ||
Revision as of 15:44, 13 November 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Repaglinide is an antidiabetic agent that is FDA approved for the {{{indicationType}}} of type 2 diabetes mellitus. Common adverse reactions include hypoglycemia, diarrhea, arthralgia, headache, sinusitis, upper respiratory infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Type 2 Diabetes Mellitus
- Starting Dose
- For patients not previously treated or whose HbA1c is < 8%, the starting dose should be 0.5 mg with each meal. For patients previously treated with blood glucose-lowering drugs and whose HbA1c is ≥ 8%, the initial dose is 1 or 2 mg with each meal preprandially.
- Dose Adjustment
- Dosing adjustments should be determined by blood glucose response, usually fasting blood glucose. Postprandial glucose levels testing may be clinically helpful in patients whose pre-meal blood glucose levels are satisfactory but whose overall glycemic control (HbA1c) is inadequate. The preprandial dose should be doubled up to 4 mg with each meal until satisfactory blood glucose response is achieved. At least one week should elapse to assess response after each dose adjustment.
- The recommended dose range is 0.5 mg to 4 mg taken with meals. PRANDIN may be dosed preprandially 2, 3, or 4 times a day in response to changes in the patient’s meal pattern. The maximum recommended daily dose is 16 mg.
- Patient Management
- Long-term efficacy should be monitored by measurement of HbA1c levels approximately every 3 months. Failure to follow an appropriate dosage regimen may precipitate hypoglycemia or hyperglycemia. Patients who do not adhere to their prescribed dietary and drug regimen are more prone to exhibit unsatisfactory response to therapy including hypoglycemia. When hypoglycemia occurs in patients taking a combination of PRANDIN and a thiazolidinedione or PRANDIN and metformin, the dose of PRANDIN should be reduced.
- Patients Receiving Other Oral Hypoglycemic Agents
- When PRANDIN is used to replace therapy with other oral hypoglycemic agents, PRANDIN may be started on the day after the final dose is given. Patients should then be observed carefully for hypoglycemia due to potential overlapping of drug effects. When transferred from longer half-life sulfonylurea agents (e.g., chlorpropamide) to repaglinide, close monitoring may be indicated for up to one week or longer.
- Combination Therapy
- If PRANDIN monotherapy does not result in adequate glycemic control, metformin or a thiazolidinedione may be added. If metformin or thiazolidinedione monotherapy does not provide adequate control, PRANDIN may be added. The starting dose and dose adjustments for PRANDIN combination therapy is the same as for PRANDIN monotherapy. The dose of each drug should be carefully adjusted to determine the minimal dose required to achieve the desired pharmacologic effect. Failure to do so could result in an increase in the incidence of hypoglycemic episodes. Appropriate monitoring of FPG and HbA1c measurements should be used to ensure that the patient is not subjected to excessive drug exposure or increased probability of secondary drug failure.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Repaglinide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Repaglinide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Repaglinide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Repaglinide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Repaglinide in pediatric patients.
Contraindications
- Diabetic ketoacidosis, with or without coma. This condition should be treated with insulin.
- Type 1 diabetes.
- Co-administration of gemfibrozil.
- Known hypersensitivity to the drug or its inactive ingredients.
Warnings
Precautions
- General:
- PRANDIN is not indicated for use in combination with NPH-insulin.
- Macrovascular Outcomes:
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with PRANDIN or any other anti-diabetic drug.
- All oral blood glucose-lowering drugs including repaglinide are capable of producing hypoglycemia. Proper patient selection, dosage, and instructions to the patients are important to avoid hypoglycemic episodes. Hepatic insufficiency may cause elevated repaglinide blood levels and may diminish gluconeogenic capacity, both of which increase the risk of serious hypoglycemia. Elderly, debilitated, or malnourished patients, and those with adrenal, pituitary, hepatic, or severe renal insufficiency may be particularly susceptible to the hypoglycemic action of glucose-lowering drugs.
- Hypoglycemia may be difficult to recognize in the elderly and in people taking beta-adrenergic blocking drugs. Hypoglycemia is more likely to occur when caloric intake is deficient, after severe or prolonged exercise, when alcohol is ingested, or when more than one glucose-lowering drug is used.
- The frequency of hypoglycemia is greater in patients with type 2 diabetes who have not been previously treated with oral blood glucose-lowering drugs (naïve) or whose HbA1c is less than 8%. PRANDIN should be administered with meals to lessen the risk of hypoglycemia.
- Loss of Control of Blood Glucose: When a patient stabilized on any diabetic regimen is exposed to stress such as fever, trauma, infection, or surgery, a loss of glycemic control may occur. At such times, it may be necessary to discontinue PRANDIN and administer insulin. The effectiveness of any hypoglycemic drug in lowering blood glucose to a desired level decreases in many patients over a period of time, which may be due to progression of the severity of diabetes or to diminished responsiveness to the drug. This phenomenon is known as secondary failure, to distinguish it from primary failure in which the drug is ineffective in an individual patient when the drug is first given. Adequate adjustment of dose and adherence to diet should be assessed before classifying a patient as a secondary failure.
Adverse Reactions
Clinical Trials Experience
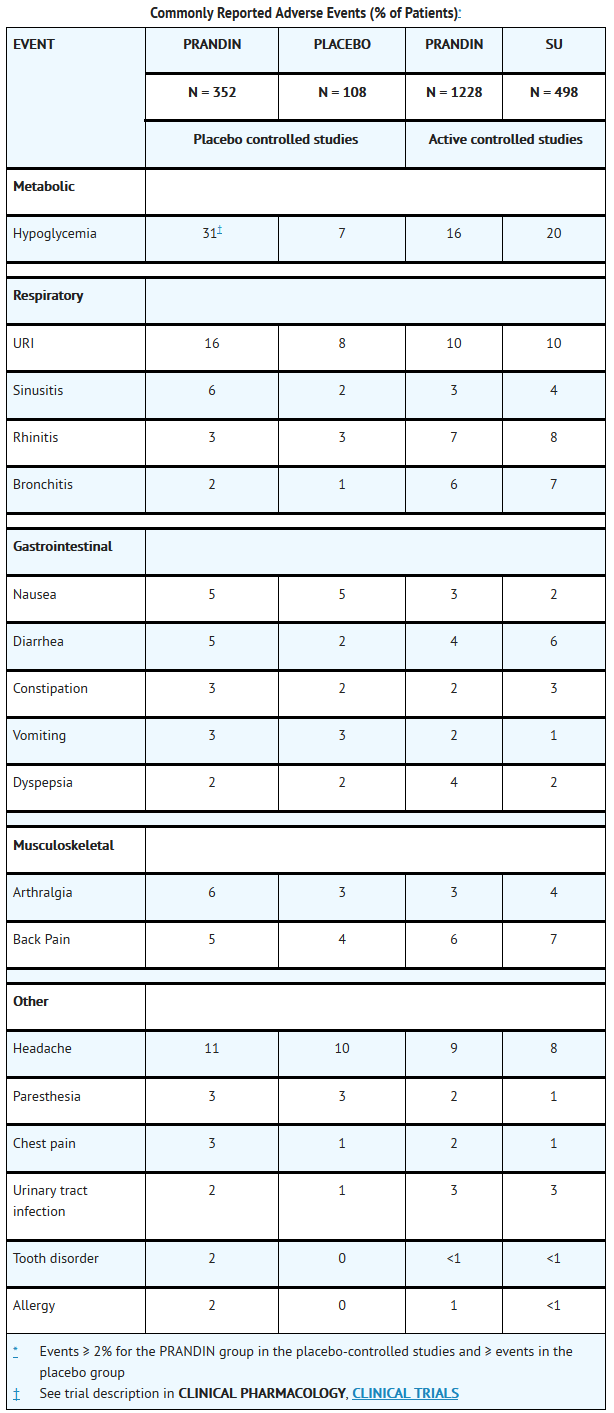
- PRANDIN has been administered to 2931 individuals during clinical trials. Approximately 1500 of these individuals with type 2 diabetes have been treated for at least 3 months, 1000 for at least 6 months, and 800 for at least 1 year. The majority of these individuals (1228) received PRANDIN in one of five 1-year, active-controlled trials. The comparator drugs in these 1-year trials were oral sulfonylurea drugs (SU) including glyburide and glipizide. Over one year, 13% of PRANDIN patients were discontinued due to adverse events, as were 14% of SU patients. The most common adverse events leading to withdrawal were hyperglycemia, hypoglycemia, and related symptoms. Mild or moderate hypoglycemia occurred in 16% of PRANDIN patients, 20% of glyburide patients, and 19% of glipizide patients.
- The table below lists common adverse events for PRANDIN patients compared to both placebo (in trials 12 to 24 weeks duration) and to glyburide and glipizide in one year trials. The adverse event profile of PRANDIN was generally comparable to that for sulfonylurea drugs (SU).
T1
- Cardiovascular Events
- In one-year trials comparing PRANDIN to sulfonylurea drugs, the incidence of angina was comparable (1.8%) for both treatments, with an incidence of chest pain of 1.8% for PRANDIN and 1.0% for sulfonylureas. The incidence of other selected cardiovascular events (hypertension, abnormal EKG, myocardial infarction, arrhythmias, and palpitations) was ≤ 1% and not different between PRANDIN and the comparator drugs.
- The incidence of total serious cardiovascular adverse events, including ischemia, was higher for repaglinide (4%) than for sulfonylurea drugs (3%) in controlled comparator clinical trials. In 1-year controlled trials, PRANDIN treatment was not associated with excess mortality when compared to the rates observed with other oral hypoglycemic agent therapies.
T2
- Seven controlled clinical trials included PRANDIN combination therapy with NPH-insulin (n=431), insulin formulations alone (n=388) or other combinations (sulfonylurea plus NPH-insulin or PRANDIN plus metformin) (n=120). There were six serious adverse events of myocardial ischemia in patients treated with PRANDIN plus NPH-insulin from two studies, and one event in patients using insulin formulations alone from another study.
- Infrequent Adverse Events (<1% of Patients)
- Less common adverse clinical or laboratory events observed in clinical trials included elevated liver enzymes, thrombocytopenia, leukopenia, and anaphylactoid reactions.
- Although no causal relationship with repaglinide has been established, postmarketing experience includes reports of the following rare adverse events: alopecia, hemolytic anemia, pancreatitis, Stevens-Johnson Syndrome, and severe hepatic dysfunction including jaundice and hepatitis.
- Combination Therapy with Thiazolidinediones
- During 24-week treatment clinical trials of PRANDIN-rosiglitazone or PRANDIN-pioglitazone combination therapy (a total of 250 patients in combination therapy), hypoglycemia (blood glucose < 50 mg/dL) occurred in 7% of combination therapy patients in comparison to 7% for PRANDIN monotherapy, and 2% for thiazolidinedione monotherapy.
- Peripheral edema was reported in 12 out of 250 PRANDIN-thiazolidinedione combination therapy patients and 3 out of 124 thiazolidinedione monotherapy patients, with no cases reported in these trials for PRANDIN monotherapy. When corrected for dropout rates of the treatment groups, the percentage of patients having events of peripheral edema per 24 weeks of treatment were 5% for PRANDIN-thiazolidinedione combination therapy, and 4% for thiazolidinedione monotherapy. There were reports in 2 of 250 patients (0.8%) treated with PRANDIN-thiazolidinedione therapy of episodes of edema with congestive heart failure. Both patients had a prior history of coronary artery disease and recovered after treatment with diuretic agents. No comparable cases in the monotherapy treatment groups were reported.
- Mean change in weight from baseline was +4.9 kg for PRANDIN-thiazolidinedione therapy. There were no patients on PRANDIN-thiazolidinedione combination therapy who had elevations of liver transaminases (defined as 3 times the upper limit of normal levels).
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Repaglinide in the drug label.
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Teratogenic Effects
- Safety in pregnant women has not been established. Repaglinide was not teratogenic in rats or rabbits at doses 40 times (rats) and approximately 0.8 times (rabbit) clinical exposure (on a mg/m2 basis) throughout pregnancy. Because animal reproduction studies are not always predictive of human response, PRANDIN should be used during pregnancy only if it is clearly needed.
- Because recent information suggests that abnormal blood glucose levels during pregnancy are associated with a higher incidence of congenital abnormalities, many experts recommend that insulin be used during pregnancy to maintain blood glucose levels as close to normal as possible.
- Nonteratogenic Effects
- Offspring of rat dams exposed to repaglinide at 15 times clinical exposure on a mg/m2 basis during days 17 to 22 of gestation and during lactation developed nonteratogenic skeletal deformities consisting of shortening, thickening, and bending of the humerus during the postnatal period. This effect was not seen at doses up to 2.5 times clinical exposure (on a mg/m2 basis) on days 1 to 22 of pregnancy or at higher doses given during days 1 to 16 of pregnancy. Relevant human exposure has not occurred to date and therefore the safety of PRANDIN administration throughout pregnancy or lactation cannot be established.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Repaglinide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Repaglinide during labor and delivery.
Nursing Mothers
- In rat reproduction studies, measurable levels of repaglinide were detected in the breast milk of the dams and lowered blood glucose levels were observed in the pups. Cross fostering studies indicated that skeletal changes (see Nonteratogenic Effects) could be induced in control pups nursed by treated dams, although this occurred to a lesser degree than those pups treated in utero. Although it is not known whether repaglinide is excreted in human milk some oral agents are known to be excreted by this route. Because the potential for hypoglycemia in nursing infants may exist, and because of the effects on nursing animals, a decision should be made as to whether PRANDIN should be discontinued in nursing mothers, or if mothers should discontinue nursing. If PRANDIN is discontinued and if diet alone is inadequate for controlling blood glucose, insulin therapy should be considered.
Pediatric Use
- No studies have been performed in pediatric patients.
Geriatic Use
- In repaglinide clinical studies of 24 weeks or greater duration, 415 patients were over 65 years of age. In one-year, active-controlled trials, no differences were seen in effectiveness or adverse events between these subjects and those less than 65 other than the expected age-related increase in cardiovascular events observed for PRANDIN and comparator drugs. There was no increase in frequency or severity of hypoglycemia in older subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals to PRANDIN therapy cannot be ruled out.
Gender
There is no FDA guidance on the use of Repaglinide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Repaglinide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Repaglinide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Repaglinide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Repaglinide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Repaglinide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Repaglinide in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Repaglinide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- In a clinical trial, patients received increasing doses of PRANDIN up to 80 mg a day for 14 days. There were few adverse effects other than those associated with the intended effect of lowering blood glucose. Hypoglycemia did not occur when meals were given with these high doses.
Management
- Hypoglycemic symptoms without loss of consciousness or neurologic findings should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring may continue until the physician is assured that the patient is out of danger. Patients should be closely monitored for a minimum of 24 to 48 hours, since hypoglycemia may recur after apparent clinical recovery. There is no evidence that repaglinide is dialyzable using hemodialysis.
- Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given a rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of more dilute (10%) glucose solution at a rate that will maintain the blood glucose at a level above 100 mg/dL.
Chronic Overdose
There is limited information regarding Chronic Overdose of Repaglinide in the drug label.
Pharmacology
There is limited information regarding Repaglinide Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Repaglinide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Repaglinide in the drug label.
Nonclinical Toxicology
- Long-term carcinogenicity studies were performed for 104 weeks at doses up to and including 120 mg/kg body weight/day (rats) and 500 mg/kg body weight/day (mice) or approximately 60 and 125 times clinical exposure, respectively, on a mg/m2 basis. No evidence of carcinogenicity was found in mice or female rats. In male rats, there was an increased incidence of benign adenomas of the thyroid and liver. The relevance of these findings to humans is unclear. The no-effect doses for these observations in male rats were 30 mg/kg body weight/day for thyroid tumors and 60 mg/kg body weight/day for liver tumors, which are over 15 and 30 times, respectively, clinical exposure on a mg/m2 basis.
- Repaglinide was non-genotoxic in a battery of in vivo and in vitro studies: Bacterial mutagenesis (Ames test), in vitro forward cell mutation assay in V79 cells (HGPRT), in vitro chromosomal aberration assay in human lymphocytes, unscheduled and replicating DNA synthesis in rat liver, and in vivo mouse and rat micronucleus tests.
- Fertility of male and female rats was unaffected by repaglinide administration at doses up to 80 mg/kg body weight/day (females) and 300 mg/kg body weight/day (males); over 40 times clinical exposure on a mg/m2 basis.
Clinical Studies
There is limited information regarding Clinical Studies of Repaglinide in the drug label.
How Supplied
Storage
There is limited information regarding Repaglinide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Repaglinide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Repaglinide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be informed of the potential risks and advantages of PRANDIN and of alternative modes of therapy. They should also be informed about the importance of adherence to dietary instructions, of a regular exercise program, and of regular testing of blood glucose and HbA1c. The risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development and concomitant administration of other glucose-lowering drugs should be explained to patients and responsible family members. Primary and secondary failure should also be explained.
- Patients should be instructed to take PRANDIN before meals (2, 3, or 4 times a day preprandially). Doses are usually taken within 15 minutes of the meal but time may vary from immediately preceding the meal to as long as 30 minutes before the meal. Patients who skip a meal (or add an extra meal) should be instructed to skip (or add) a dose for that meal.
Precautions with Alcohol
- Alcohol-Repaglinide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Repaglinide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Repaglinide |Label Name=Repaglinide11.png
}}
{{#subobject:
|Label Page=Repaglinide |Label Name=Repaglinide11.png
}}