Relapsing fever pathophysiology
|
Relapsing fever Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Relapsing fever pathophysiology On the Web |
|
American Roentgen Ray Society Images of Relapsing fever pathophysiology |
|
Risk calculators and risk factors for Relapsing fever pathophysiology |
keywords:VMP:Variable membrane proteins
Overview
Borrelia is usually transmitted via the tick bite or body louse to the human host. After entering the bloodstream, spirochetes replicate extracellularly and remain predominantly in the plasma space. Patients generally remain asymptomatic until high-level spirochetemia (104-108 organisms m!) develops, at which time symptoms begin abruptly. Organisms are cleared predominantly by opsonizing antibodies with the resolution of symptoms ( afebrile period), followed several days or weeks later by the reemergence of a new antigenic strain, high-level spirochetemia, and recurrence of symptoms. There are multiple genes in the spirochete encoding variable membrane proteins( VMPs). These VMPs determine the antigenic serotype of the organism. At any given time, each spirochete has VMP genes that are expressed and others that are silent. An antigenic switch occurs when a given VMP gene transposes from silent to an expressed locus. This cyclical process of initially effective immune response followed by antigenic variation and immunologic escape is responsible for the relapsing nature of this illness.
Pathophysiology
- Borrelia is usually transmitted via the tick bite or body louse to the human host.
Cyclical process
Incubation period
Incubation period = time from tick bite to illness
- 7 days, range 2 to 18 days
Symptomatic period
Symptomatic period= Length of illness = time from symptom onset to resolution of symptoms
- 3 days, range 2 to 7 days
- In LBRF, the fever usually lasts 3-6 days and is usually followed by a single, milder episode. In TRBF, multiple episodes of fever occur, and each may last up to 3 days.
- Following transmission, the spirochetes replicate extracellularly and remain predominantly the plasma space. Some organisms are found within circulating polymorphonuclear cells or reticuloendothelial cells, or occasionally can be found in tissues such as the liver, spleen, kidney, heart, or brain, with foci of perivascular inflammation, hemorrhage, and infarction at autopsy.
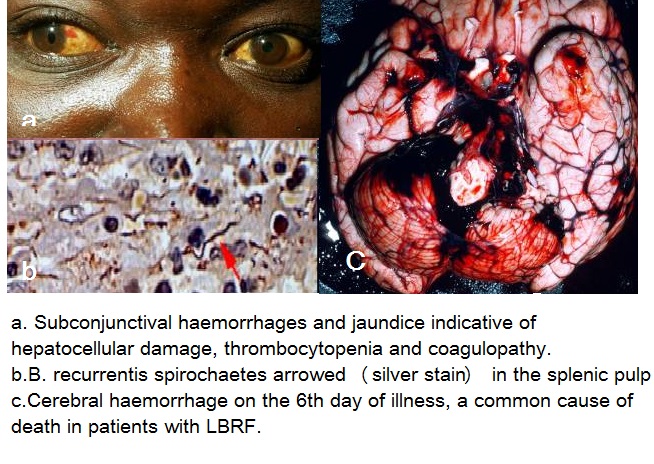
- Most cases eventually resolve spontaneously. But during episodes of spirochetemia, the organisms may invade the brain, eye, inner ear, heart, liver. Splenic abscesses and infarcts, and hemorrhages within the central nervous system, a perivascular histiocytic interstitial myocarditis, conduction defects, arrhythmias, and myocardial failure, splenic rupture with massive hemorrhage, cerebral hemorrhage, and hepatic failure are seen in LBRF which can result in sudden death.Hepatitis with patchy hemorrhages and necrosis, meningitis, and perisplenitis, thrombi in small vessels also are found. Thrombi are occasionally found occluding small vessels.[1]CNS involvement is more common in TBRF than LBRF, however eschars, ARDS, cranial nerve palsies, focal neurologic deficits, uveitis, iritis or iridocyclitis, splenic rupture and myocarditis may be seen in both TBRF and LBRF.[2][3]Most information on the pathophysiologic aspects of fatal cases comes from autopsy data of louse-borne disease in humans or experimental animals.[3].
Afebrile period
Afebrile period= Length of time before reoccurrence = time from the resolution of symptoms to reoccurrence of symptoms
- 7 days, range 4 to 14 days
- Organisms are cleared predominantly by opsonizing antibodies with the resolution of symptoms ( afebrile period), followed several days or weeks later by the reemergence of a new antigenic strain, high-level spirochetemia, when high-level spirochetemia (104-108 organisms m!) develops, at which time symptoms begin abruptly.
Crisis
- AS mentioned before, most cases eventually resolve spontaneously. Occasionally, the crisis occurs after the resolution, which is a classic series of stages that a person will go through and may develop cerebral edema with seizures, cardiac failure, or death in up to 10% of people.
Genetics
The development of the cyclical process is the result of antigenic variation and subsequent immunologic escape:
- There are multiple genes in the spirochete encoding variable membrane proteins( VMPs) which are the outer membrane of spirochetes contains surface proteins. Attacks of relapsing fever end abruptly when specific bactericidal immunoglobulin M antibodies generated by the B1b cell subset lyse spirochaetes in the blood, independently of complement and T cells. These VMPs determine the antigenic serotype of the organism and at any given time, each spirochete has VMP genes that are expressed and others that are silent. An antigenic switch occurs when a given VMP gene transposes from silent to an expressed locus. This mechanism of antigenic variation presumably leads to immunologic escape and relapse. Because of the organism’s ability to undergo antigenic variation, several serotypes may be present in the bloodstream at one time.
References
- ↑ Warrell DA (January 2019). "Louse-borne relapsing fever (Borrelia recurrentis infection)". Epidemiol. Infect. 147: e106. doi:10.1017/S0950268819000116. PMC 6518520 Check
|pmc=value (help). PMID 30869050. - ↑ Blevins SM, Greenfield RA, Bronze MS (July 2008). "Blood smear analysis in babesiosis, ehrlichiosis, relapsing fever, malaria, and Chagas disease". Cleve Clin J Med. 75 (7): 521–30. doi:10.3949/ccjm.75.7.521. PMID 18646588.
- ↑ 3.0 3.1 Dworkin MS, Schwan TG, Anderson DE, Borchardt SM (September 2008). "Tick-borne relapsing fever". Infect. Dis. Clin. North Am. 22 (3): 449–68, viii. doi:10.1016/j.idc.2008.03.006. PMC 3725823. PMID 18755384.