Pyridoxine (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pyridoxine (oral) is a nutritive agent of the vitamin B family that is FDA approved for the treatment of inadequate dietary intake. Common adverse reactions include paresthesia and somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- As a dietary supplement for adults, take one tablet daily with a full glass of water, preferably after a meal.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pyridoxine (oral) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pyridoxine (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Pyridoxine (oral) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pyridoxine (oral) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pyridoxine (oral) in pediatric patients.
Contraindications
There is limited information regarding Pyridoxine (oral) Contraindications in the drug label.
Warnings
- Keep out of reach of children.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Pyridoxine (oral) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Pyridoxine (oral) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Pyridoxine (oral) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Pyridoxine (oral) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pyridoxine (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pyridoxine (oral) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Pyridoxine (oral) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Pyridoxine (oral) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Pyridoxine (oral) in geriatric settings.
Gender
There is no FDA guidance on the use of Pyridoxine (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pyridoxine (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pyridoxine (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pyridoxine (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pyridoxine (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pyridoxine (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Pyridoxine (oral) Administration in the drug label.
Monitoring
There is limited information regarding Pyridoxine (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Pyridoxine (oral) and IV administrations.
Overdosage
There is limited information regarding Pyridoxine (oral) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Natural substances that have vitamin B6 activity are pyridoxine in plants and pyridoxal or pyridoxamine in animals. All 3 are converted to pyridoxal phosphate by the enzyme pyridoxal kinase. The physiologically active forms of vitamin B6 are pyridoxal phosphate (codecarboxylase) and pyridoxamine phosphate. Riboflavin is required for the conversion of pyridoxine phosphate to pyridoxal phosphate.
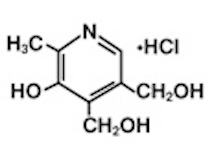
Structure
- The chemical name is 2-methyl-3-hydroxy-4,5-bis (hydroxymethyl) pyridine hydrochloride. The structural formula is:
Pharmacodynamics
There is limited information regarding Pyridoxine (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Pyridoxine (oral) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Pyridoxine (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Pyridoxine (oral) Clinical Studies in the drug label.
How Supplied

Storage
- Store in a cool, dry place, tightly closed.
Images
Drug Images
{{#ask: Page Name::Pyridoxine (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - BOTTLE LABEL 25 MG
NDC 61748-092-30
PYRIDOXINE SUPPLEMENT25 mg USP (Vitamin B-6) 30 TABLETS

{{#ask: Label Page::Pyridoxine (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Pyridoxine (oral) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Pyridoxine (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Pyridoxine (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.