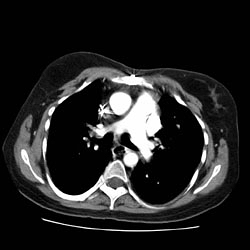
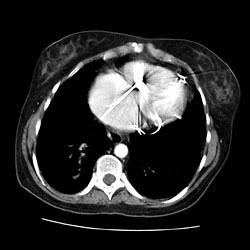
Pulmonary nodule CT
|
Pulmonary Nodule Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Pulmonary nodule CT On the Web |
|
American Roentgen Ray Society Images of Pulmonary nodule CT |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Recommendations for management based on appearance
Solid nodules
For response by physicians to lung nodules found on CT scans, the clinical practice guidelines by the American College of Chest Physicians (ACCP) recommends[1]:
- If less than 8 mm, use guidelines in table below by the Fleischner society (see table below).
- For nodules greater than 8 mm in diameter, assess the patients risk of complications from thoracic surgery:
- If high risk for complications of surgery, obtain non-surgical biopsy
- If low to moderate risk for complications of surgery, assess probability of cancer by a validated calculation. The model developed at the Mayo Clinic has been the most extensively validated[2]. An open-source version is available online.
| Nodule Size (mm) | Low† risk patients | High‡ risk patients |
|---|---|---|
| <= 4 | No follow-up needed. | Follow-up at 12 months. If no change, no further imaging needed. |
| >4 - 6 | Follow-up at 12 months. If no change, no further imaging needed. | Initial follow-up CT at 6 -12 months and then at 18 - 24 months if no change. |
| >6 - 8 | Initial follow-up CT at 6 -12 months and then at 18 - 24 months if no change. | Initial follow-up CT at 3 - 6 months and then at 9 -12 and 24 months if no change. |
| >8 | Follow-up CTs at around 3, 9, and 24 months. Dynamic contrast enhanced CT, PET, and/or biopsy. | Same at for low risk patients. |
| † Low risk patients: Minimal or absent history of smoking and of other known risk factors. ‡ High risk patients: History of smoking or of other known risk factors. | ||
Subsolid nodules
The ACCP suggests subsolid nodules may require an extended duration of surveillance for growth or signs of a solid component as these are often premalignant or malignant[1]. The Fleischner society has published guidelines for the management of subsolid nodules[4].
Example images
CT

Halo Sign
- The halo sign refers to a zone of ground-glass attenuation surrounding a pulmonary nodule or mass on CT images.
- The presence of a halo of ground-glass opacity or ground-glass attenuation is usually associated with hemorrhagic nodules.
- In severely neutropenic patients, the halo sign is highly suggestive of infection by an angioinvasive fungus, most commonly Aspergillus.
- Vascular invasion by this fungus results in thrombosis of small- to medium-sized vessels, which causes ischemic necrosis.
- At pathologic examination, the nodules represent foci of infarction, and the halo of ground-glass attenuation results from alveolar hemorrhage.
- Although it is less common, the halo sign may also be observed in nonhemorrhagic nodules, in which case either tumor cells or inflammatory infiltrate account for the halo of ground-glass attenuation.
References
- ↑ 1.0 1.1 Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP; et al. (2013). "Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines". Chest. 143 (5 Suppl): e93S–120S. doi:10.1378/chest.12-2351. PMC 3749714. PMID 23649456.
- ↑ Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES (1997). "The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules". Arch Intern Med. 157 (8): 849–55. PMID 9129544.
- ↑ MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP; et al. (2005). "Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society". Radiology. 237 (2): 395–400. doi:10.1148/radiol.2372041887. PMID 16244247.
- ↑ Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM; et al. (2013). "Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society". Radiology. 266 (1): 304–17. doi:10.1148/radiol.12120628. PMID 23070270.