Potassium perchlorate
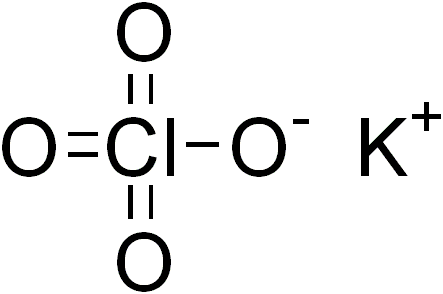
| |
| Template:Chembox image sbs cell | |
| File:Potassium perchlorate 200g.jpg | |
| Names | |
|---|---|
| Other names
Potassium chlorate(VII); Perchloric acid, potassium salt; peroidin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| ClKO4 | |
| Molar mass | 138.54 g·mol−1 |
| Hazards | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Potassium perchlorate is the inorganic salt with the chemical formula KClO4. Like other perchlorates, this salt is a strong oxidizer although it usually reacts very slowly with organic substances. This usually obtained as a colorless, crystalline solid is a common oxidizer used in fireworks, ammunition percussion caps, explosive primers, and is used variously in propellants, flash compositions, stars, and sparklers. It has been used as a solid rocket propellant, although in that application it has mostly been replaced by the higher performance ammonium perchlorate. KClO4 has the lowest solubility of the alkali metal perchlorates (1.5 g in 100 mL of water at 25 °C).[1]
Production
KClO4 is prepared industrially by treating an aqueous solution of sodium perchlorate with KCl. This single precipitation reaction exploits the low solubility of KClO4, which is about 100 times less than the solubility of NaClO4 (209.6 g/100 mL at 25 °C).[6] It can also be produced by the reaction of perchloric acid with potassium hydroxide, however this is not used widely, due to the dangers of perchloric acid.
Oxidizing properties
KClO4 is an oxidizer in the sense that it exothermically transfers oxygen to combustible materials, greatly increasing their rate of combustion relative to that in air. Thus, with glucose it gives carbon dioxide:
- 3 KClO4 + C6H12O6 → 6 H2O + 6 CO2 + 3 KCl
The conversion of solid glucose into hot gaseous CO2 is the basis of the explosive force of this and other such mixtures. Even with cane sugar, KClO4 yields a low explosive, provided the necessary confinement. Otherwise such mixtures simply deflagrate with an intense purple flame characteristic of potassium. Flash compositions used in firecrackers usually consist of fine aluminium powder mixed with potassium perchlorate.
As an oxidizer, potassium perchlorate can be used safely in the presence of sulfur, whereas potassium chlorate cannot. The greater reactivity of chlorate is typical – perchlorates are kinetically poorer oxidants. Chlorate produces chloric acid, which is highly unstable and can lead to premature ignition of the composition. Correspondingly, perchloric acid is quite stable.[7]
In medicine
Potassium perchlorate can be used as an antithyroid agent used to treat hyperthyroidism, usually in combination with one other medication. This application exploits the similar ionic radii and hydrophilicity of perchlorate and iodide.
Other Uses
Since 2005, a cartridge with potassium perchlorate mixed with anthracene and sulfur is used for generating the black smoke signalling the failure of reaching a two-thirds majority needed for the election of new pope by a papal conclave.
References
- ↑ 1.0 1.1 "Potassium Perchlorate MSDS". J.T. Baker. 2007-02-16. Retrieved 2007-12-10.
- ↑ 2.0 2.1 2.2 2.3 http://chemister.ru/Database/properties-en.php?dbid=1&id=519
- ↑ http://www.solubilityofthings.com/water/ions_solubility/ksp_chart.php
- ↑ Benenson, Walter; Stöcker, Horst. Handbook of Physics. Springer. p. 780. ISBN 0387952691.
|access-date=requires|url=(help) - ↑ 5.0 5.1 5.2 5.3 Sigma-Aldrich Co., Potassium perchlorate. Retrieved on 2014-05-27.
- ↑ Helmut Vogt, Jan Balej, John E. Bennett, Peter Wintzer, Saeed Akbar Sheikh, Patrizio Gallone "Chlorine Oxides and Chlorine Oxygen Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_483
- ↑ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
External links
| Wikimedia Commons has media related to Potassium perchlorate. |
- Pages with script errors
- Pages using citations with accessdate and no URL
- Pages with broken file links
- Articles without KEGG source
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Chembox
- Chembox having DSD data
- Chembox having GHS data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Commons category link is defined as the pagename
- Commons category link is on Wikidata using P373
- Potassium compounds
- Perchlorates
- Oxidizing agents