Poractant Alfa
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Poractant Alfa is a lung surfactant that is FDA approved for the treatment of Respiratory Distress Syndrome (RDS) in premature infants. Common adverse reactions include bradycardia, hypotension, endotracheal tube blockage, and oxygen desaturation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Indication and Dosage of Poractant Alfa in adult patients.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Poractant Alfa in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Poractant Alfa in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indication
CUROSURF® (poractant alfa) Intratracheal Suspension is indicated for the rescue treatment of Respiratory Distress Syndrome (RDS) in premature infants. CUROSURF reduces mortality and pneumothoraces associated with RDS.
Dosage
The initial recommended dose is 2.5 mL/kg birth weight (see Table 1), administered as one or two aliquots depending upon the instillation procedure.
Up to two repeat doses of 1.25 mL/kg birth weight each may be administered at approximately 12-hour intervals in infants who remain intubated and in whom RDS is considered responsible for their persisting or deteriorating respiratory status. The maximum recommended total dosage (sum of the initial and up to two repeat doses) is 5 mL/kg.
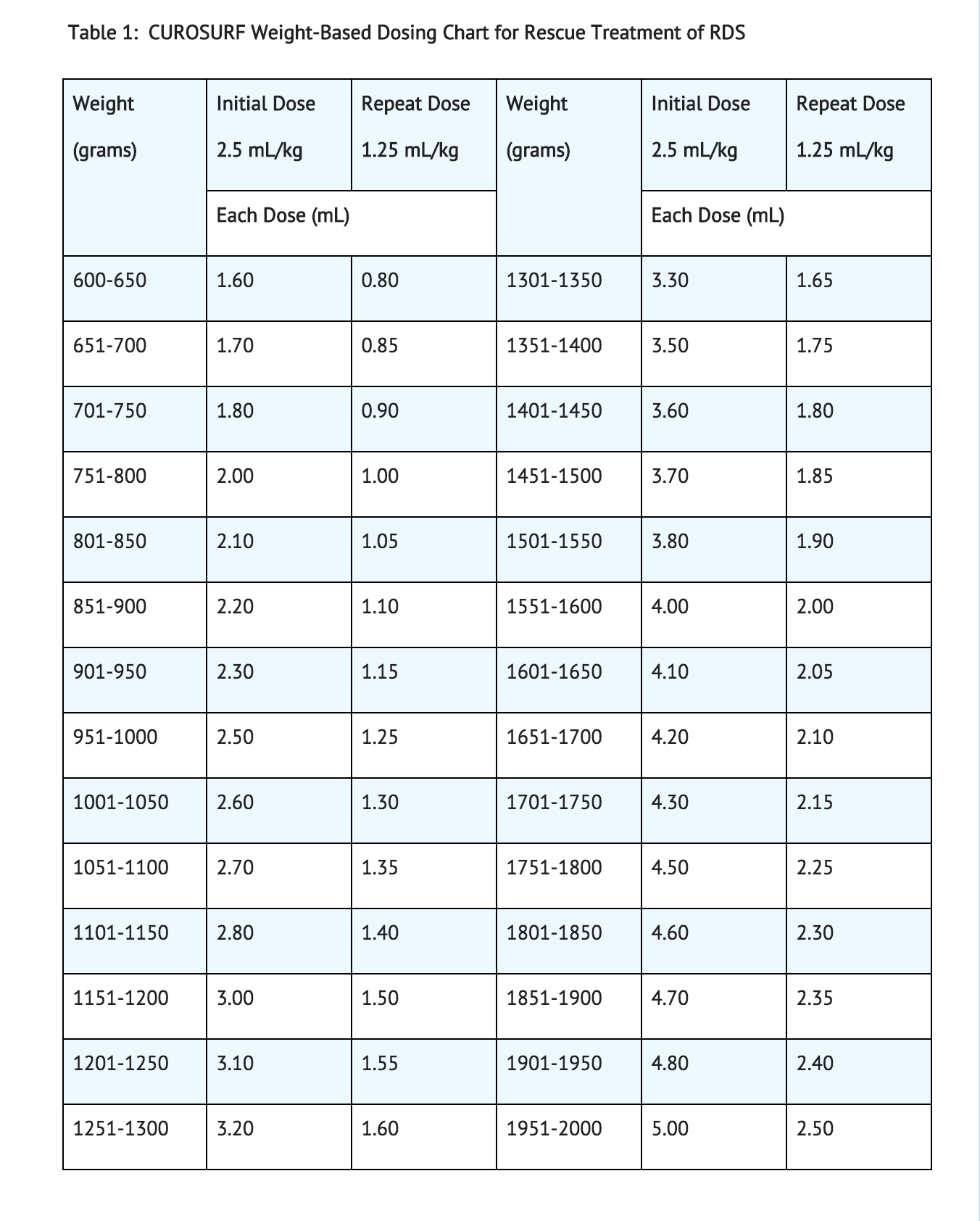
Preparation of the CUROSURF Suspension
- Remove the vial of CUROSURF suspension from a refrigerator at +2 to +8°C (36 to 46°F) and slowly warm the vial to room temperature before use.
- Visually inspect the CUROSURF suspension for discoloration prior to administration. *The color of the CUROSURF suspension should be white to creamy white. Discard the CUROSURF vial if the suspension is discolored.
- Gently turn the vial upside-down, in order to obtain a uniform suspension.
- DO NOT SHAKE.
- Locate the notch (FLIP UP) on the colored plastic cap and lift the notch and pull upwards.
- Pull the plastic cap with the aluminum portion downwards.
- Remove the whole ring by pulling off the aluminum wrapper.
- Remove the rubber cap to extract content.
- Unopened, unused vials of CUROSURF suspension that have warmed to room temperature can be returned to refrigerated storage within 24 hours for future use.
- Do not warm to room temperature and return to refrigerated storage more than once.
- Protect from light.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Poractant Alfa in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Poractant Alfa in pediatric patients.
Contraindications
None.
Warnings
Acute Changes in Oxygenation and Lung Compliance
The administration of exogenous surfactants, including CUROSURF, can rapidly affect oxygenation and lung compliance. Therefore, infants receiving CUROSURF should receive frequent clinical and laboratory assessments so that oxygen and ventilatory support can be modified to respond to respiratory changes. CUROSURF should only be administered by those trained and experienced in the care, resuscitation, and stabilization of pre-term infants.
Administration-Related Adverse Reactions
Transient adverse reactions associated with administration of CUROSURF include bradycardia, hypotension, endotracheal tube blockage, and oxygen desaturation. These events require stopping CUROSURF administration and taking appropriate measures to alleviate the condition. After the patient is stable, dosing may proceed with appropriate monitoring.
Adverse Reactions
Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Studies in Premature Infants with Respiratory Distress Syndrome
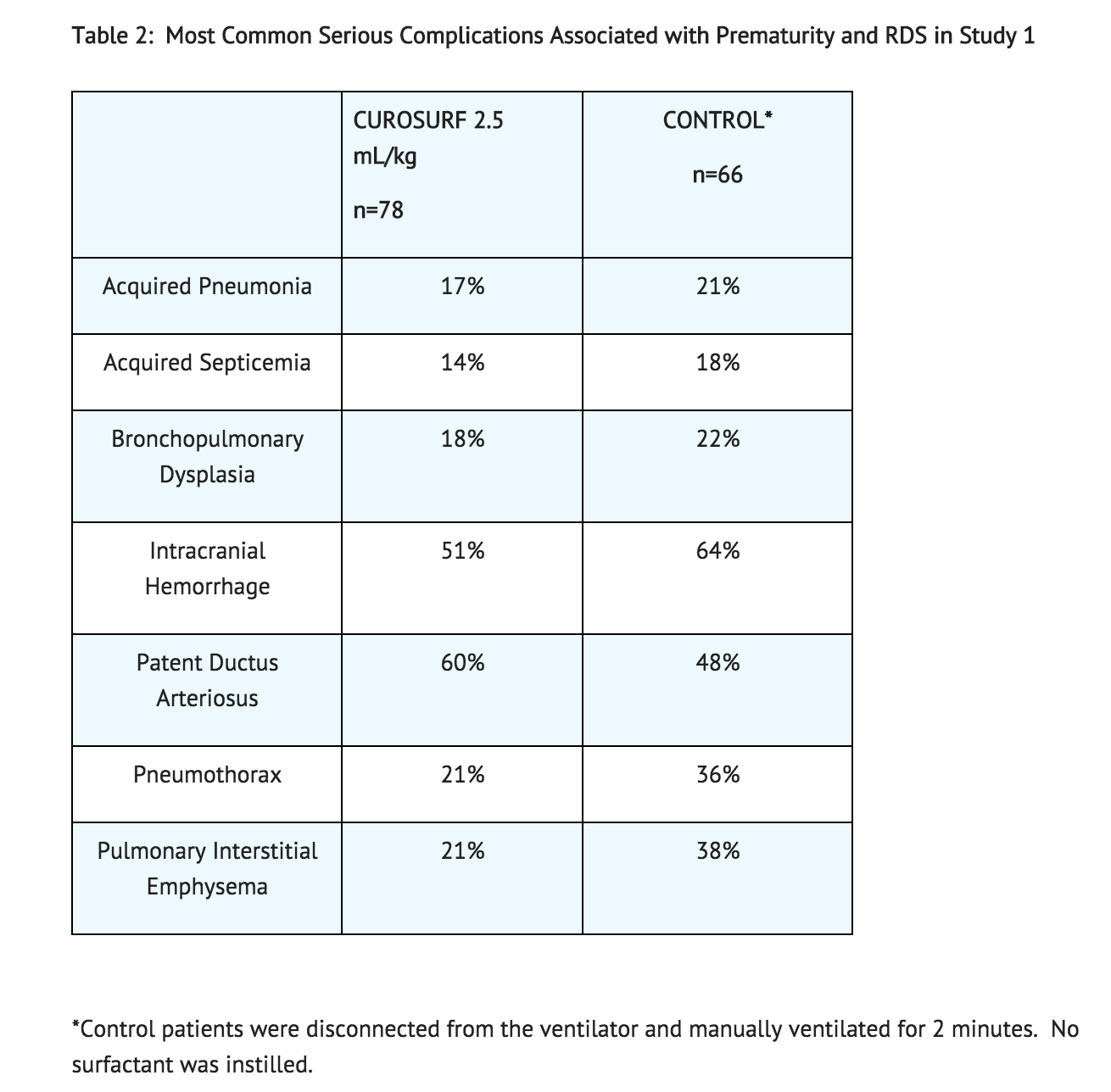
The safety data described below reflect exposure to CUROSURF at a single dose of 2.5 mL/kg (200 mg/kg), in 78 infants of 700-2000 grams birth weight with RDS requiring mechanical ventilation and a FiO2 ≥ 0.60 (Study 1). A total of 144 infants were studied after RDS developed and before 15 hours of age; 78 infants received CUROSURF 2.5 mL/kg single dose (200 mg/kg), and 66 infants received control treatment (disconnection from the ventilator and manual ventilation for 2 minutes).
Transient adverse effects seen with the administration of CUROSURF included bradycardia, hypotension, endotracheal tube blockage, and oxygen desaturation. The rates of the most common serious complications associated with prematurity and RDS observed in Study 1 are shown in Table 2.
Seventy-six infants (45 treated with CUROSURF) from study 1 were evaluated at 1 year of age and 73 infants (44 treated with CUROSURF) were evaluated at 2 years of age to assess for potential long-term adverse reactions. Data from follow-up evaluations for weight and length, persistent respiratory symptoms, incidence of cerebral palsy, visual impairment, or auditory impairment was similar between treatment groups. In 16 patients (10 treated with CUROSURF and 6 controls) evaluated at 5.5 years of age, the developmental quotient, derived using the Griffiths Mental Developmental Scales, was similar between groups.
Immunogenicity
Immunological studies have not demonstrated differences in levels of surfactant-anti-surfactant immune complexes and anti-CUROSURF antibodies between patients treated with CUROSURF and patients who received control treatment.
Postmarketing Experience
Pulmonary hemorrhage, a known complication of premature birth and very low birth-weight, has been reported both in clinical trials with CUROSURF and in postmarketing adverse event reports in infants who had received CUROSURF.
Drug Interactions
There is limited information regarding Drug Interactions of Poractant Alfa.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Poractant Alfa in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Poractant Alfa in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Poractant Alfa during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Poractant Alfa in women who are nursing.
Pediatric Use
CUROSURF is indicated for the rescue treatment, including the reduction of mortality and pneumothoraces, of Respiratory Distress Syndrome (RDS) in premature infants.
The safety and efficacy of CUROSURF in the treatment of full term infants or older pediatric patients with respiratory failure has not been established.
Geriatic Use
There is no FDA guidance on the use of Poractant Alfa in geriatric settings.
Gender
There is no FDA guidance on the use of Poractant Alfa with respect to specific gender populations.
Race
There is no FDA guidance on the use of Poractant Alfa with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Poractant Alfa in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Poractant Alfa in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Poractant Alfa in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Poractant Alfa in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Poractant Alfa Administration in the drug label.
Monitoring
There is limited information regarding Poractant Alfa Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Poractant Alfa and IV administrations.
Overdosage
There have been no reports of overdosage following the administration of CUROSURF.
In the event of accidental overdosage, and if there are clear clinical effects on the infant's respiration, ventilation, or oxygenation, aspirate as much of the suspension as possible and provide the infant with supportive treatment, with particular attention to fluid and electrolyte balance.
Pharmacology
There is limited information regarding Poractant Alfa Pharmacology in the drug label.
Mechanism of Action
Endogenous pulmonary surfactant reduces surface tension at the air-liquid interface of the alveoli during ventilation and stabilizes the alveoli against collapse at resting transpulmonary pressures. A deficiency of pulmonary surfactant in preterm infants results in Respiratory Distress Syndrome (RDS) characterized by poor lung expansion, inadequate gas exchange, and a gradual collapse of the lungs (atelectasis).
CUROSURF compensates for the deficiency of surfactant and restores surface activity to the lungs of these infants.
Structure
There is limited information regarding Poractant Alfa Structure in the drug label.
Pharmacodynamics
In vitro - CUROSURF lowers minimum surface tension to ≤ 4mN/m as measured by the Wilhelmy Balance System.
Pharmacokinetics
CUROSURF is administered directly to the lung, where biophysical effects occur at the alveolar surface. No human pharmacokinetic studies have been performed to characterize the absorption, biotransformation, or elimination of CUROSURF.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to assess potential carcinogenic effects of CUROSURF have not been conducted.
Poractant alfa was negative for genotoxicity in the following assays: bacterial reverse mutation assay (Ames test), gene mutation assay in Chinese hamster V79 cells, chromosomal aberration assay in Chinese hamster ovary cells, unscheduled DNA synthesis in HELA S3 cells, and in vivo mouse micronucleus assay.
No studies to assess reproductive effects of CUROSURF have been performed.
Clinical Studies
There is limited information regarding Poractant Alfa Clinical Studies in the drug label.
How Supplied
There is limited information regarding Poractant Alfa How Supplied in the drug label.
Storage
There is limited information regarding Poractant Alfa Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Poractant Alfa |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Poractant Alfa |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Poractant Alfa Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Poractant Alfa interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Poractant Alfa Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Poractant Alfa Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.