Polycystic kidney disease: Difference between revisions
No edit summary |
|||
| Line 50: | Line 50: | ||
Image:autorecessive.jpg|ARPKD is inherited in an [[Recessive gene|autosomal recessive]] pattern. | Image:autorecessive.jpg|ARPKD is inherited in an [[Recessive gene|autosomal recessive]] pattern. | ||
</gallery> | </gallery> | ||
Image:Autosomal-recessive-polycystic-kidney-disease-001.jpg | |||
Image:Autosomal-recessive-polycystic-kidney-disease-002.jpg | |||
Image:Autosomal-recessive-polycystic-kidney-disease-003.jpg | |||
===Other types=== | ===Other types=== | ||
Revision as of 18:27, 7 March 2009
Template:DiseaseDisorder infobox Template:Search infobox Steven C. Campbell, M.D., Ph.D.
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Polycystic kidney disease (PKD, also known as polycystic kidney syndrome) is a progressive, genetic disorder of the kidneys. It occurs in humans and other organisms. PKD is characterized by the presence of multiple cysts (hence, "polycystic") in both kidneys. The disease can also damage the liver, pancreas, and rarely, the heart and brain. The two major forms of polycystic kidney disease are distinguished by their patterns of inheritance.
Autosomal dominant polycystic kidney disease (ADPKD) is generally a late-onset disorder characterized by progressive cyst development and bilaterally enlarged kidneys with multiple cysts. Kidney manifestations in this disorder include renal function abnormalities, hypertension, renal pain, and renal insufficiency. Approximately 50% of patients with ADPKD have end stage renal disease (ESRD) by the age of 60. ADPKD is a systemic disease with cysts in other organs such as the liver (which may lead to cirrhosis), seminal vesicles, pancreas, and arachnoid mater and non-cystic abnormalities such as intracranial aneurysms and dolichoectasias, dilatation of the aortic root and dissection of the thoracic aorta, mitral valve prolapse, and abdominal wall hernias.
Initial simian and human symptoms are hypertension, fatigue, and mild to severe back or flank pain and urinary tract infections. The disease often leads to chronic renal failure and may result in total loss of kidney function, known as end stage renal disease (ESRD), which requires some form of renal replacement therapy (e.g. dialysis).
Autosomal recessive polycystic kidney disease (ARPKD) is much rarer than ADPKD and is often fatal in utero or during the first month of life. The signs and symptoms of the condition are usually apparent at birth or in early infancy.
Genetics
The disease exists both in an autosomal recessive and an autosomal dominant form.
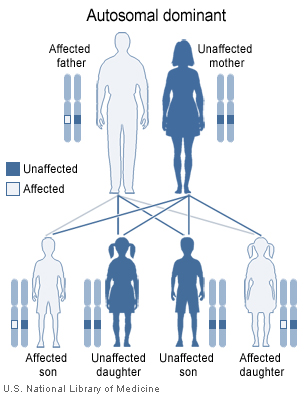
Autosomal dominant form
The autosomal dominant form, called ADPKD (autosomal dominant PKD or "Adult-onset PKD") is much more common but less severe. In 85% of patients, ADPKD is caused by mutations in the gene PKD1 on chromosome 16 (TRPP1); in 15% of patients mutations in PKD2 (TRPP2) are causative. A third locus PKD3 is the cause of a very small percentage of cases.
-
ADPKD is inherited in an autosomal dominant pattern.
Autosomal recessive form
The recessive form, called ARPKD (autosomal recessive polycystic kidney disease) is the less common variant. Mutations in the PKHD1 (chromosomal locus 6p12.2) cause ARPKD.
-
ARPKD is inherited in an autosomal recessive pattern.
Image:Autosomal-recessive-polycystic-kidney-disease-001.jpg Image:Autosomal-recessive-polycystic-kidney-disease-002.jpg Image:Autosomal-recessive-polycystic-kidney-disease-003.jpg
Other types
A small number of families with polycystic kidney disease do not have apparent mutations in any of the three known genes. An unidentified gene or genes may also be responsible for this disease. In this case, the disease is designated "PKD3".
Epidemiology
Polycystic kidney disease is the most common life-threatening genetic disease, affecting approximately 7 million people worldwide. Autosomal dominant polycystic kidney disease affects up to 1 in 1000 people, while the autosomal recessive type is estimated to occur in approximately 1 in 20,000 people.[1][2]
Pathophysiology
Recent studies in fundamental cell biology of cilia/flagella using experimental model organisms like the green algae Chlamydomonas, the round worm Caenorhabditis elegans and the mouse Mus musculus have shed light on how PKD develops in patients. All cilia and flagella are constructed and maintained, including localizing of proteins inserted into ciliary and flagellar membranes, by the process of intraflagellar transport. Environmental sensing and cellular signaling pathways initiated from proteins inserted into ciliary/flagellar membranes are thought to be critical for normal renal cell development and functioning. Membrane proteins which function in developmental and physiological environmental sensing and intracellular signalling are sorted to and localized to the cilia in renal epithelial cells by intraflagellar transport. These epithelial cells line the lumen of the urinary collecting ducts and sense the flow of urine. Failure in flow-sensing signaling results in programmed cell death or apoptosis of these renal epithelial cells producing the characteristic multiple cysts of PKD. PKD may result from mutations of signaling and environmental sensing proteins, or failure in intraflagellar transport. Two PKD genes, PKD1 and PKD2, encode membrane proteins which localize to a non-motile cilium on the renal tube cell. Polycystin-2 encoded by PKD2 gene is a calcium channel which allows extracellular calcium ions to enter the cell. Polycystin-1, encoded by PKD1 gene, is thought to be associated with polycystin-2 protein and regulate its channel activity. The calcium ions are important cellular messengers which, in turn, trigger complicated biochemical pathways which lead to quiescence and differentiation. Malfunctions of polycystin-1 or polycystin-2 proteins, defects in the assembly of the cilium on the renal tube cell, failures in targeting these two proteins to the cilium, and deregulations of calcium signaling all likely cause the occurrence of PKD.
PKD and the "two hit" hypothesis:
The two hit hypothesis (aka Knudson hypothesis ) is often used to explain the manifestation of polycystic kidney disease later in life even though the mutation is present at birth. This term is borrowed from cancer research stating that both copies of the gene present in the genome have to be "silenced" before cancer manifests itself (in Knudson's case the silenced gene was Rb1). In ADPKD the original "hit" is congenital (in either the PKD1 or PKD2 genes) and the subsequent "hit" occurs later in life as the cells grow and divide. The two hit hypothesis as it relates to PKD was originally proposed by Reeders in 1992.[3] Support for this hypothesis comes from the fact that ARPKD patients develop disease at birth, and somatic mutations in the "normal" copy of PKD1 or PKD2 have been found in cyst-lining epithelia
Diagnosis
A definite diagnosis of ADPKD relies on imaging or molecular genetic testing. The sensitivity of testing is nearly 100% for all patients with ADPKD who are age 30 years or older and for younger patients with PKD1 mutations; these criteria are only 67% sensitive for patients with PKD2 mutations who are younger than age 30 years. Large echogenic kidneys without distinct macroscopic cysts in an infant/child at 50% risk for ADPKD are diagnostic. In the absence of a family history of ADPKD, the presence of bilateral renal enlargement and cysts, with or without the presence of hepatic cysts, and the absence of other manifestations suggestive of a different renal cystic disease provide presumptive, but not definite, evidence for the diagnosis.
Molecular genetic testing by linkage analysis or direct mutation screening is available clinically; however, genetic heterogeneity is a significant complication to molecular genetic testing. Sometimes a relatively large number of affected family members need to be tested in order to establish which one of the two possible genes is responsible within each family. The large size and complexity of PKD1 and PKD2 genes, as well as marked allelic heterogeneity, present obstacles to molecular testing by direct DNA analysis. In the research setting, mutation detection rates of 50-75% have been obtained for PKD1 and ~75% for PKD2. Clinical testing of the PKD1 and PKD2 genes by direct sequence analysis is now available, with a detection rate for disease-causing mutations of 50-70%.
Genetic counseling may be helpful for families at risk for polycystic kidney disease.
Treatment
Although a cure for PKD is not available, treatment can ease the symptoms and prolong life.
- Pain: Over-the-counter pain medications, such as paracetamol can relieve pain. For most but not all cases of severe pain, surgery to shrink cysts can relieve pain in the back and flanks. However, surgery provides only temporary relief and usually does not slow the disease's progression toward kidney failure.
- Urinary tract infections: Patients with PKD tend to have frequent urinary tract infections, which can be treated with antibiotics. Early treatment is important, because infection can spread from the urinary tract to the cysts in the kidneys. Cyst infections are difficult to treat because many antibiotics do not penetrate into the cysts. However, some antibiotics are effective.
- High blood pressure: Keeping blood pressure under control can slow the effects of PKD. Lifestyle changes and various medications can lower high blood pressure.
- End-stage renal disease: There are two options for replacing kidney functions: dialysis or transplantation. Healthy (non-PKD) kidneys transplanted into PKD patients do not develop cysts.
Pathological Findings
-
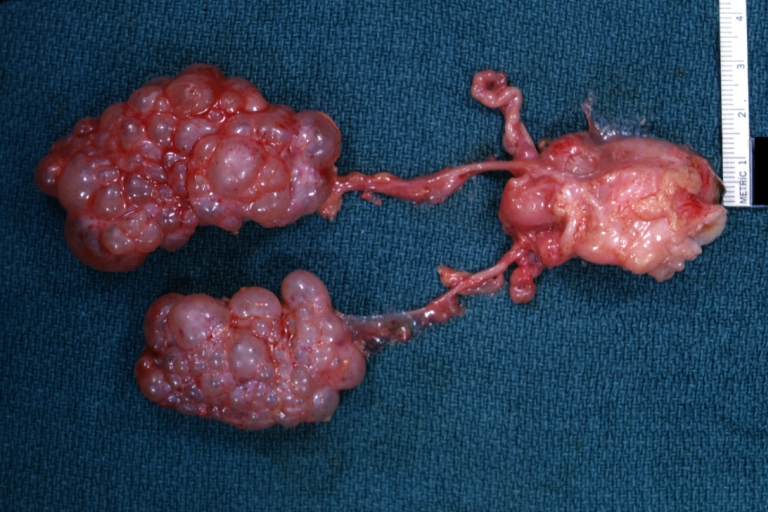
Infantile polycystic disease: Gross natural color view of both kidneys with ureters and uterus (very good example)
Adult type Polycystic kidney disease
<youtube v=6Ws9cfsjZIk/>
Resources
The PKD Foundation is the only non-profit organization worldwide dedicated solely to PKD research. Parent of two children with ARPKD blog: www.kidneysandeyes.com
References
- ↑ DALGAARD OZ (1957). "Bilateral polycystic disease of the kidneys; a follow-up of two hundred and eighty-four patients and their families". Acta Med. Scand. Suppl. 328: 1–255. PMID 13469269.
- ↑ Zerres K, Mücher G, Becker J; et al. (1998). "Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): molecular genetics, clinical experience, and fetal morphology". Am. J. Med. Genet. 76 (2): 137–44. PMID 9511976.
- ↑ Reeders ST (1992). "Multilocus polycystic disease". Nat. Genet. 1 (4): 235–7. doi:10.1038/ng0792-235. PMID 1338768.
- Nauli SM, Zhou J (2004). "Polycystins and mechanosensation in renal and nodal cilia". Bioessays. 26 (8): 844–56. doi:10.1002/bies.20069. PMID 15273987.
- Grantham JJ, Torres VE, Chapman AB; et al. (2006). "Volume progression in polycystic kidney disease". N. Engl. J. Med. 354 (20): 2122–30. doi:10.1056/NEJMoa054341. PMID 16707749.
External links
- Photo of a polycystic kidney
- http://kidney.niddk.nih.gov/kudiseases/pubs/polycystic/index.htm
- http://www.ncbi.nlm.nih.gov/disease/PKD.html
de:Zystenniere nl:Polycysteuze nieren sv:Polycystisk njursjukdom