Pimavanserin: Difference between revisions
Martin Nino (talk | contribs) (Created page with "{{DrugProjectFormSinglePage |authorTag=Martin Nino, M.D. [mailto:martinnino@hotmail.com] |genericName=Pimavanserin |aOrAn=an |drugClass=atypical antip...") |
Martin Nino (talk | contribs) No edit summary |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=patients with [[hallucinations]] and [[delusions]] associated with [[Parkinson's disease]] [[psychosis]] | |indication=patients with [[hallucinations]] and [[delusions]] associated with [[Parkinson's disease]] [[psychosis]] | ||
|adverseReactions= | |adverseReactions=[[peripheral edema]] and [[confusional state]] (≥5% and twice the rate of [[placebo]]). | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|blackBoxWarningTitle=<span style="color:#FF0000;"> INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED | |blackBoxWarningTitle=<span style="color:#FF0000;"> INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS</span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;"> | |blackBoxWarningBody=<i><span style="color:#FF0000;"> | ||
Elderly patients with dementia-related | Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Pimavanserin is not approved for the treatment of patients with dementia-related psychosis unrelated to the hallucinations and delusions associated with Parkinson's disease psychosis.</span></i> | ||
|fdaLIADAdult=======Indications====== | |||
Pimavanserin is indicated for the treatment of [[hallucinations]] and [[delusions]] associated with [[Parkinson's disease]] [[psychosis]]. | |||
======Dosage====== | |||
:*'''General Dosing Information''' | |||
The recommended dose of Pimavanserin is 34 mg, taken orally as two 17 mg strength tablets once daily, without [[titration]]. | |||
Pimavanserin can be taken with or without food. | |||
:*'''Dosage Modifications for Concomitant Use with [[CYP3A4]] Inhibitors and Inducers''' | |||
::*Coadministration with Strong CYP3A4 Inhibitors | |||
The recommended dose of Pimavanserin when coadministered with strong CYP3A4 inhibitors (e.g., [[ketoconazole]]) is 17 mg, taken orally as one tablet once daily. | |||
::*Coadministration with Strong CYP3A4 Inducers | |||
Monitor patients for reduced efficacy if Pimavanserin is used concomitantly with strong CYP3A4 inducers; an increase in Pimavanserin dosage may be needed. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Pimavanserin in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Pimavanserin in adult patients. | |||
|fdaLIADPed=Safety and effectiveness have not been established in pediatric patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Pimavanserin in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Pimavanserin in pediatric patients. | |||
|contraindications=None | |||
|warnings=======Increased Mortality in Elderly Patients with Dementia-Related Psychosis====== | |||
[[Antipsychotic drugs]] increase the all-cause risk of death in elderly patients with [[dementia]]-related [[psychosis]]. Analyses of 17 dementia-related psychosis [[placebo]]-controlled [[trials]] (modal duration of 10 weeks and largely in patients taking atypical antipsychotic drugs) revealed a risk of death in the drug-treated patients of between 1.6- to 1.7-times that in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in placebo-treated patients. | |||
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., [[heart failure]], [[sudden death]]) or infectious (e.g., pneumonia) in nature. Pimavanserin is not approved for the treatment of patients with dementia-related psychosis unrelated to the [[hallucinations]] and [[delusions]] associated with P[[arkinson's disease]] [[psychosis]]. | |||
======[[QT Interval]] Prolongation====== | |||
Pimavanserin prolongs the QT interval. The use of Pimavanserin should be avoided in patients with known QT prolongation or in combination with other drugs known to prolong QT interval including Class 1A [[antiarrhythmics]] (e.g., [[quinidine]], [[procainamide]]) or Class 3 [[antiarrhythmics]] (e.g., [[amiodarone]], [[sotalol]]), certain [[antipsychotic]] medications (e.g., [[ziprasidone]], [[chlorpromazine]], [[thioridazine]]), and certain [[antibiotics]] (e.g., [[gatifloxacin]], [[moxifloxacin]]). Pimavanserin should also be avoided in patients with a history of cardiac [[arrhythmias]], as well as other circumstances that may increase the risk of the occurrence of [[torsade de pointes]] and/or [[sudden death]], including symptomatic [[bradycardia]], [[hypokalemia]] or [[hypomagnesemia]], and the presence of congenital prolongation of the QT interval. | |||
|clinicalTrials=The following serious adverse reactions are discussed elsewhere in the labeling: | |||
:*Increased Mortality in Elderly Patients with [[Dementia]]-Related [[Psychosis]] | |||
:*[[QT Interval]] Prolongation | |||
Because [[clinical trials]] are conducted under widely varying conditions, [[adverse reaction]] rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
The clinical trial database for Pimavanserin consists of over 1200 subjects and patients exposed to one or more doses of Pimavanserin. Of these, 616 were patients with [[hallucinations]] and [[delusions]] associated with [[Parkinson's disease]] [[psychosis]] (PDP). In the [[placebo]]-controlled setting, the majority of experience in patients comes from studies evaluating once-daily Pimavanserin doses of 34 mg (N=202) compared to placebo (N=231) for up to 6 weeks. In the controlled trial setting, the study population was approximately 64% male and 91% Caucasian, and the mean age was about 71 years at study entry. Additional clinical trial experience in patients with hallucinations and delusions associated with PDP comes from two open-label, safety extension studies (total N=497). The majority of patients receiving long-term treatment received 34 mg once-daily (N=459). Over 300 patients have been treated for more than 6 months; over 270 have been treated for at least 12 months; and over 150 have been treated for at least 24 months. | |||
The following adverse reactions are based on the 6-week, placebo-controlled studies in which Pimavanserin was administered once daily to patients with [[hallucinations]] and [[delusions]] associated with PDP. | |||
Common Adverse Reactions (incidence ≥5% and at least twice the rate of [[placebo]]): [[peripheral edema]] (7% Pimavanserin 34 mg vs. 2% placebo) and [[confusional state]] (6% Pimavanserin 34 mg vs. 3% placebo). | |||
'''Adverse Reactions Leading to Discontinuation of Treatment''' | |||
A total of 8% (16/202) of Pimavanserin 34 mg-treated patients and 4% (10/231) of [[placebo]]-treated patients discontinued because of adverse reactions. The adverse reactions that occurred in more than one patient and with an incidence at least twice that of placebo were [[hallucination]] (2% Pimavanserin vs. <1% placebo), [[urinary tract infection]] (1% Pimavanserin vs. <1% placebo), and [[fatigue]] (1% Pimavanserin vs. 0% placebo). | |||
Adverse reactions that occurred in 6-week, [[placebo]]-controlled studies and that were reported at an incidence of ≥2% and >placebo are presented in Table 1. | |||
:*'''Table 1 Adverse Reactions in Placebo-Controlled Studies of 6-Week Treatment Duration and Reported in ≥2% and >Placebo''' | |||
[[File:table1_pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
'''Adverse Reactions in Demographic Subgroups''' | |||
Examination of population subgroups in the 6-week, [[placebo]]-controlled studies did not reveal any differences in safety on the basis of age (≤75 vs. >75 years) or sex. Because the study population was predominantly Caucasian (91%; consistent with reported demographics for PD/PDP), racial or ethnic differences in the safety profile of Pimavanserin could not be assessed. In addition, in the 6-week, placebo-controlled studies, no clinically relevant differences in the incidence of adverse reactions were observed among those with a [[Mini-Mental State Examination]] (MMSE) score at entry of <25 versus those with scores ≥25. | |||
|drugInteractions=======Drugs Having Clinically Important Interactions with Pimavanserin====== | |||
:*'''Table 2 Clinically Important Drug Interactions with Pimavanserin''' | |||
[[File:table2_pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
======Drugs Having No Clinically Important Interactions with Pimavanserin====== | |||
Based on [[pharmacokinetic]] studies, no dosage adjustment of [[carbidopa]]/[[levodopa]] is required when administered concomitantly with Pimavanserin. | |||
|useInPregnancyFDA=*Risk Summary | |||
There are no data on Pimavanserin use in pregnant women that would allow assessment of the drug-associated risk of major [[congenital malformations]] or [[miscarriage]]. In animal reproduction studies, no adverse developmental effects were seen when Pimavanserin was administered orally to rats or rabbits during the period of [[organogenesis]] at doses up to 10- or 12-times the maximum recommended human dose (MRHD) of 34 mg/day, respectively. Administration of Pimavanserinto pregnant rats during pregnancy and lactation resulted in maternal toxicity and lower pup survival and body weight at doses which are 2-times the MRHD of 34 mg/day. | |||
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. | |||
*Data | |||
:*Animal Data | |||
Pimavanserin was not [[teratogenic]] in pregnant rats when administered during the period of [[organogenesis]] at oral doses of 0.9, 8.5, and 51 mg/kg/day, which are 0.2- and 10-times the maximum recommended human dose (MRHD) of 34 mg/day based on [[AUC]] at mid and high doses, respectively. Maternal [[toxicity]] included reduction in body weight and food consumption at the highest dose. | |||
Administration of Pimavanserin to pregnant rats during pregnancy and lactation at oral doses of 8.5, 26, and 51 mg/kg/day, which are 0.14- to 14-times the MRHD of 34 mg/day based on [[AUC]], caused maternal [[toxicity]], including mortality, clinical signs including [[dehydration]], [[hunched posture]], and [[rales]], and decreases in body weight, and/or food consumption at doses ≥26 mg/kg/day (2-times the MRHD based on AUC). At these maternally toxic doses there was a decrease in pup survival, reduced litter size, and reduced pup weights, and food consumption. Pimavanserin had no effect on sexual maturation, [[neurobehavioral]] function including learning and memory, or reproductive function in the first generation pups up to 14-times the MRHD of 34 mg/day based on [[AUC]]. | |||
Pimavanserin was not [[teratogenic]] in pregnant rabbits during the period of [[organogenesis]] at oral doses of 4.3, 43, and 85 mg/kg/day, which are 0.2- to 12-times the MRHD of 34 mg/day based on [[AUC]]. Maternal [[toxicity]], including mortality, clinical signs of [[dyspnea]] and [[rales]], decreases in body weight and/or food consumption, and [[abortions]] occurred at doses 12-times the MRHD of 34 mg/day based on AUC. | |||
|useInNursing=There is no information regarding the presence of Pimavanserin in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Pimavanserin and any potential adverse effects on the breastfed infant from Pimavanserin or from the underlying maternal condition. | |||
|useInPed=Safety and effectiveness of Pimavanserin have not been established in pediatric patients. | |||
|useInGeri=No dose adjustment is required for elderly patients. | |||
[[Parkinson's disease]] is a disorder occurring primarily in individuals over 55 years of age. The mean age of patients enrolled in the 6-week clinical studies with Pimavanserin was 71 years, with 49% 65-75 years old and 31% >75 years old. In the pooled population of patients enrolled in 6-week, [[placebo]]-controlled studies (N=614), 27% had MMSE scores from 21 to 24 compared to 73% with scores ≥25. No clinically meaningful differences in safety or effectiveness were noted between these two groups. | |||
|useInRenalImpair=No dosage adjustment for Pimavanserin is needed in patients with mild to moderate ([[CrCL]] ≥30 mL/min, [[Cockcroft-Gault]]) [[renal impairment]]. | |||
Use of Pimavanserin is not recommended in patients with severe [[renal impairment]] ([[CrCL]] <30 mL/min, [[Cockcroft-Gault]]). Pimavanserin has not been evaluated in this patient population. | |||
|useInHepaticImpair=Use of Pimavanserin is not recommended in patients with [[hepatic impairment]]. Pimavanserin has not been evaluated in this patient population. | |||
|administration=The recommended dose of Pimavanserin is 34 mg, taken orally as two 17 mg strength tablets once daily, without [[titration]]. | |||
Pimavanserin can be taken with or without food. | |||
|overdose='''Human Experience''' | |||
The pre-marketing [[clinical trials]] involving Pimavanserin in approximately 1200 subjects and patients do not provide information regarding symptoms with overdose. In healthy subject studies, dose-limiting nausea and vomiting were observed. | |||
'''Management of Overdose''' | |||
There are no known specific [[antidotes]] for Pimavanserin. In managing overdose, cardiovascular monitoring should commence immediately and should include continuous [[ECG]] monitoring to detect possible [[arrhythmias]]. If [[antiarrhythmic]] therapy is administered, [[disopyramide]], [[procainamide]], and [[quinidine]] should not be used, as they have the potential for [[QT]]-prolonging effects that might be additive to those of Pimavanserin. Consider the long plasma [[half-life]] of Pimavanserin (about 57 hours) and the possibility of multiple drug involvement. Consult a Certified Poison Control Center (1-800-222-1222) for up-to-date guidance and advice. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 448227829 | |||
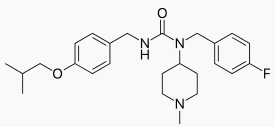
| IUPAC_name = ''N''-(4-fluorophenylmethyl)-''N''-(1-methylpiperidin-4-yl)-''N'''-(4-(2-methylpropyloxy)phenylmethyl)carbamide | |||
| image = estructure_pima.png | |||
| width = 275 | |||
<!--Clinical data--> | |||
| tradename = Nuplazid | |||
| pregnancy_category = | |||
| legal_US = Rx-only | |||
| routes_of_administration = Oral ([[Tablet (pharmacy)|tablets]]) | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = 94–97%<ref name = Rev>{{cite journal|title=Pimavanserin for the treatment of Parkinson’s disease psychosis|author=Friedman, JH|journal=Expert Opinion on Pharmacotherapy|date=October 2013|volume=14|issue=14|pages=1969–1975|doi=10.1517/14656566.2013.819345|pmid=24016069}}</ref> | |||
| metabolism = [[Liver|Hepatic]] ([[CYP3A4]], [[CYP3A5]], [[CYP2J2]])<ref name="PI">{{cite web|title=Nuplazid (pimavanserin) Tablets, for Oral Use. U.S. Full Prescribing Information|url=https://www.nuplazid.com/Prescribinginformation.pdf|publisher=ACADIA Pharmaceuticals Inc.|accessdate=1 May 2016}}</ref> | |||
| elimination_half-life = 54–56 hours<ref name = Rev/> | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 706779-91-1 | |||
| CAS_supplemental = <br />706782-28-7 ([[tartrate]]) | |||
| ATC_prefix = N05 | |||
| ATC_suffix = AX17 | |||
| PubChem = 10071196 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = JZ963P0DIK | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ChemSpiderID = 8246736 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 2111101 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 133017 | |||
| KEGG_Ref = {{keggcite|changed|kegg}} | |||
| KEGG = D08969 | |||
| DrugBank_Ref = {{drugbankcite|changed|drugbank}} | |||
| DrugBank = DB05316 | |||
<!--Chemical data--> | |||
| C = 25 | H = 34 | F = 1 | N = 3 | O = 2 | |||
| molecular_weight = 427.553 g/mol | |||
| smiles = CC(C)COc3ccc(cc3)CNC(=O)N(C(CC2)CCN2C)Cc(cc1)ccc1F | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChI = 1S/C25H34FN3O2/c1-19(2)18-31-24-10-6-20(7-11-24)16-27-25(30)29(23-12-14-28(3)15-13-23)17-21-4-8-22(26)9-5-21/h4-11,19,23H,12-18H2,1-3H3,(H,27,30) | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChIKey = RKEWSXXUOLRFBX-UHFFFAOYSA-N | |||
| synonyms = ACP-103 | |||
}} | |||
|mechAction=The mechanism of action of Pimavanserin in the treatment of [[hallucinations]] and [[delusions]] associated with [[Parkinson's disease]] [[psychosis]] is unknown. However, the effect of Pimavanserin could be mediated through a combination of [[inverse agonist]] and [[antagonist]] activity at [[serotonin]] [[5-HT2A receptors]] and to a lesser extent at [[serotonin]] [[5-HT2C receptors]]. | |||
|PD=[[In vitro]], Pimavanserin acts as an inverse [[agonist]] and [[antagonist]] at [[serotonin]] [[5-HT2A receptors]] with high binding affinity (Ki value 0.087 nM) and at [[serotonin]] [[5-HT2C receptors]] with lower binding affinity (Ki value 0.44 nM). Pimavanserin shows low binding to [[sigma 1 receptors]] (Ki value 120 nM) and has no appreciable affinity (Ki value >300 nM), to [[serotonin]] [[5-HT2B]], [[dopaminergic]] (including D2), [[muscarinic]], [[histaminergic]], or [[adrenergic receptors]], or to [[calcium channels]]. | |||
'''Cardiac Electrophysiology''' | |||
The effect of Pimavanserin on the [[QTc interval]] was evaluated in a randomized [[placebo]]- and positive-controlled [[double-blind]], multiple-dose parallel thorough QTc study in 252 healthy subjects. A central tendency analysis of the QTc data at steady-state demonstrated that the maximum mean change from baseline (upper bound of the two-sided 90% CI) was 13.5 (16.6) msec at a dose of twice the therapeutic dose. A [[pharmacokinetic]]/ [[pharmacodynamic]] analysis with Pimavanserin suggested a concentration-dependent QTc interval prolongation in the therapeutic range. | |||
In the 6-week, [[placebo]]-controlled effectiveness studies, mean increases in [[QTc interval]] of ~5-8 msec were observed in patients receiving once-daily doses of Pimavanserin 34 mg. These data are consistent with the profile observed in a thorough QT study in healthy subjects. Sporadic QTcF values ≥500 msec and change from baseline values ≥60 msec were observed in subjects treated with Pimavanserin 34 mg; although the incidence was generally similar for Pimavanserin and [[placebo]] groups. There were no reports of [[torsade de pointes]] or any differences from placebo in the incidence of other adverse reactions associated with delayed [[ventricular repolarization]] in studies of Pimavanserin, including those patients with [[hallucinations]] and [[delusions]] associated with PDP. | |||
|PK=Pimavanserin demonstrates dose-proportional [[pharmacokinetics]] after single oral doses from 17 to 255 mg (0.5- to 7.5-times the recommended dosage). The pharmacokinetics of Pimavanserin are similar in both the study population and healthy subjects. The mean plasma [[half-lives]] for Pimavanserin and the active [[metabolite]] (N-desmethylated metabolite) are approximately 57 hours and 200 hours, respectively. | |||
'''Absorption''' | |||
The median [[Tmax]] of Pimavanserin was 6 (range 4-24) hours and was generally unaffected by dose. The [[bioavailability]] of Pimavanserin oral tablet and Pimavanserin solution was essentially identical. The formation of the major circulating N-desmethylated [[metabolite]] AC-279 (active) from Pimavanserin occurs with a median Tmax of 6 hours. | |||
Ingestion of a high-fat meal had no significant effect on rate ([[Cmax]]) and extent ([[AUC]]) of Pimavanserin exposure. Cmax decreased by about 9% while AUC increased by about 8% with a high-fat meal. | |||
'''Distribution''' | |||
Pimavanserin is highly [[protein bound]] (~95%) in human plasma. Protein binding appeared to be dose-independent and did not change significantly over dosing time from Day 1 to Day 14. Following administration of a single dose of Pimavanserin (34 mg), the mean (SD) apparent [[volume of distribution]] was 2173 (307) L. | |||
'''Elimination''' | |||
:*Metabolism | |||
Pimavanserin is predominantly metabolized by [[CYP3A4]] and [[CYP3A5]] and to a lesser extent by [[CYP2J2]], [[CYP2D6]], and various other [[CYP]] and [[FMO]] [[enzymes]]. [[CYP3A4]] is the major enzyme responsible for the formation of its major active [[metabolite]] (AC-279). Pimavanserin does not cause clinically significant CYP inhibition or induction of [[CYP3A4]]. Based on [[in vitro]] data, Pimavanserin is not an irreversible inhibitor of any of the major hepatic and intestinal human [[CYP]] enzymes involved in drug [[metabolism]] ([[CYP1A2]], [[2B6]], [[2C8]], [[2C9]], [[2C19]], [[2D6]], and [[3A4]]). | |||
Based on [[in vitro]] studies, [[transporters]] play no significant role in the disposition of Pimavanserin. | |||
AC-279 is neither a reversible or irreversible (metabolism-dependent) inhibitor of any of the major hepatic and intestinal human [[CYP]] enzymes involved in drug metabolism ([[CYP1A2]], [[2B6]], [[2C8]], [[2C9]], [[2C19]], [[2D6]], and [[3A4]]). AC-279 does not cause clinically significant [[CYP3A]] induction and is not predicted to cause induction of any other [[CYP]] enzymes involved in drug metabolism. | |||
:*Excretion | |||
Approximately 0.55% of the 34 mg oral dose of 14C-Pimavanserin was eliminated as unchanged drug in urine and 1.53% was eliminated in feces after 10 days. | |||
Less than 1% of the administered dose of Pimavanserin and its [[active metabolite]] AC-279 were recovered in urine. | |||
'''Specific Populations''' | |||
Population [[PK]] analysis indicated that exposure of Pimavanserin in patients with mild to moderate [[renal impairment]] was similar to exposure in patients with normal [[renal function]]. Age, sex, ethnicity, and weight do not have clinically relevant effect on the [[pharmacokinetics]] of Pimavanserin. | |||
Pimavanserin has not been studied in patients with severe [[renal impairment]] or mild to severe [[hepatic impairment]]. | |||
'''Drug Interaction Studies''' | |||
[[CYP3A4]] Inhibitor: [[ketoconazole]], a strong inhibitor of CYP3A4, increased Pimavanserin [[Cmax]] by 1.5-fold and [[AUC]] by 3-fold. | |||
The effect of Pimavanserin on other drugs is shown in Figure 1. | |||
:*'''Figure 1 The Effects of Pimavanserin on the [[Pharmacokinetics]] of Other Drugs''' | |||
[[File:figure1_pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
NUPLAZID: Pimavanserin's Brand name | |||
|nonClinToxic=======[[Carcinogenesis]], [[Mutagenesis]], Impairment of Fertility====== | |||
:*'''[[Carcinogenesis]]''' | |||
There was no increase in the incidence of tumors following daily oral administration of Pimavanserin to mice or rats for 2 years. Mice were administered Pimavanserin at oral doses of 2.6, 6, and 13 (males)/8.5, 21, and 43 mg/kg/day (females) which are 0.01- to 1- (males)/0.5- to 7- (females) times the MRHD of 34 mg/day based on [[AUC]]. Rats were administered Pimavanserin at oral doses of 2.6, 8.5, and 26 (males)/4.3, 13, and 43 mg/kg/day (females) which are 0.01- to 4- (males)/0.04- to 16- (females) times the MRHD of 34 mg/day based on [[AUC]]. | |||
:*'''[[Mutagenesis]]''' | |||
Pimavanserin was not mutagenic in the [[in vitro]] Ames reverse mutation test, or in the in vitro mouse lymphoma assay, and was not [[clastogenic]] in the in vivo mouse [[bone marrow]] micronucleus assay. | |||
:*'''Impairment of Fertility''' | |||
Pimavanserin was administered orally to male and female rats before mating, through mating, and up to Day 7 of gestation at doses of 8.5, 51, and 77 mg/kg/day, which are approximately 2-, 15-, and 22-times the maximum recommended human dose (MRHD) of 34 mg/day based on mg/m2, respectively. Pimavanserin had no effect on fertility or reproductive performance in male and female rats at doses up to 22-times the MRHD of 34 mg based on mg/m2. Changes in uterine parameters (decreases in the number of [[corpora lutea]], number of [[implants]], [[viable implants]], and increases in [[pre-implantation]] loss, early [[resorptions]] and [[post-implantation]] loss) occurred at the highest dose which was also a maternally toxic dose. Changes in sperm parameters (decreased density and motility) and microscopic findings of [[cytoplasmic vacuolation]] in the [[epididymis]] occurred at doses approximately 15-times the MRHD of 34 mg/day based on mg/m2. | |||
======Animal [[Toxicology]] and/or [[Pharmacology]]====== | |||
[[Phospholipidosis]] (foamy [[macrophages]] and/or [[cytoplasmic vacuolation]]) was observed in multiple tissues and organs of mice, rats, and monkeys as early as 14 days following oral daily administration of Pimavanserin. The most severely affected organs were the lungs and kidneys. The occurrence of [[phospholipidosis]] was both dose- and duration-dependent. Diffuse [[phospholipidosis]] with focal/multifocal [[chronic inflammation]] was observed in the lungs of rats treated for ≥3 months at doses ≥10-times the maximum recommended human dose (MRHD) of 34 mg/day based on [[AUC]]. As a result of chronic inflammation, [[inflammatory lung fibrosis]] was observed in rats treated for 3 and 6 months at doses ≥18-times the MRHD of 34 mg/day based on AUC. The findings in the lungs correlated with increased lung weights (up to 3-times those of controls) and respiratory-related clinical signs including [[rales]], [[labored breathing]], and [[gasping]]. [[Phospholipidosis]] in lungs of rats caused mortality at doses ≥16-times the MRHD of 34 mg/day based on AUC. The estimated No Observed Effect Level (NOEL) for chronic lung inflammation in rats is 5-fold the MRHD of 34 mg/day based on AUC. [[Phospholipidosis]] was associated with increased kidney weights and [[tubular degeneration]] in rats at doses ≥10-times the MRHD of 34 mg/day based on AUC. The relevance of these findings to human risk is not known. | |||
|clinicalStudies=The efficacy of Pimavanserin 34 mg as a treatment of [[hallucinations]] and [[delusions]] associated with [[Parkinson's disease]] [[psychosis]] was demonstrated in a 6-week, randomized, [[placebo]]-controlled, parallel-group study. In this outpatient study, 199 patients were randomized in a 1:1 ratio to Pimavanserin 34 mg or placebo once daily. Study patients (male or female and aged 40 years or older) had a diagnosis of Parkinson's disease (PD) established at least 1 year prior to study entry and had psychotic symptoms (hallucinations and/or delusions) that started after the PD diagnosis and that were severe and frequent enough to warrant treatment with an [[antipsychotic]]. At entry, patients were required to have a [[Mini-Mental State Examination]] (MMSE) score ≥21 and to be able to self-report symptoms. The majority of patients were on PD medications at entry; these medications were required to be stable for at least 30 days prior to study start and throughout the study period. | |||
The PD-adapted Scale for the Assessment of Positive Symptoms (SAPS-PD) was used to evaluate the efficacy of Pimavanserin 34 mg. SAPS-PD is a 9-item scale adapted for PD from the Hallucinations and Delusions domains of the SAPS. Each item is scored on a scale of 0-5, with 0 being none and 5 representing severe and frequent symptoms. Therefore, the SAPS-PD total score can range from 0 to 45 with higher scores reflecting greater severity of illness. A negative change in score indicates improvement. Primary efficacy was evaluated based on change from baseline to Week 6 in SAPS-PD total score. | |||
As shown in Table 3, Figure 2, and Figure 3, Pimavanserin 34 mg (n=95) was statistically significantly superior to [[placebo]] (n=90) in decreasing the frequency and/or severity of hallucinations and delusions in patients with PDP as measured by central, independent, and blinded raters using the SAPS-PD scale. An effect was seen on both the hallucinations and delusions components of the SAPS-PD. | |||
:*'''Table 3 Primary Efficacy Analysis Result Based on SAPS-PD (N=185)''' | |||
[[File:table3_pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
NUPLAZID: Pimavanserin's Brand name | |||
The effect of Pimavanserin on SAPS-PD improved through the six-week trial period, as shown in Figure 2. | |||
:*'''Figure 2 SAPS-PD Change from Baseline through 6 Weeks Total Study Treatment''' | |||
[[File:figure2_pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
NUPLAZID: Pimavanserin's Brand name | |||
:*'''Figure 3 Proportion of Patients with SAPS-PD Score Improvement at the End of Week 6 (N=185)''' | |||
[[File:figure3_pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
NUPLAZID: Pimavanserin's Brand name | |||
'''[[Motor Function]] in Patients with [[Hallucinations]] and [[Delusions]] Associated with [[Parkinson's Disease]] [[Psychosis]]''' | |||
Pimavanserin 34 mg did not show an effect compared to [[placebo]] on motor function, as measured using the Unified Parkinson's Disease Rating Scale Parts II and III (UPDRS Parts II+III) (Figure 4). A negative change in score indicates improvement. The UPDRS Parts II+III was used to assess the patient's Parkinson's disease state during the 6-week [[double-blind]] treatment period. The UPDRS score was calculated as the sum of the 40 items from activities of daily living and motor examination, with a range of 0 to 160. | |||
:*'''Figure 4 Motor Function Change from Baseline to Week 6 in UPDRS Parts II+III (LSM - SE)''' | |||
[[File:figure4_pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
NUPLAZID: Pimavanserin's Brand name | |||
|howSupplied=Pimavanserin tablets are available as: | |||
'''17 mg Tablet:''' | |||
White to off-white, round, immediate-release, film-coated tablet debossed with "P" on one side and "17" on the reverse. . Each tablet contains 20 mg of pimavanserin tartrate, which is equivalent to 17 mg of Pimavanserin free base. Inactive ingredients include pregelatinized starch, magnesium stearate, and microcrystalline cellulose. Additionally, the following inactive ingredients are present as components of the film coat: hypromellose, talc, titanium dioxide, polyethylene glycol, and saccharin sodium. | |||
Bottle of 60: NDC 63090-170-60 | |||
|storage=Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). | |||
|packLabel=[[File:pima.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo=Advise patients to inform their healthcare providers if there are any changes to their current prescription or over-the-counter medications, since there is a potential for drug interactions. | |||
|brandNames=NUPLAZID™ | |||
}} | }} | ||
Latest revision as of 13:17, 26 January 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete Boxed Warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Pimavanserin is not approved for the treatment of patients with dementia-related psychosis unrelated to the hallucinations and delusions associated with Parkinson's disease psychosis.
|
Overview
Pimavanserin is an atypical antipsychotic that is FDA approved for the treatment of patients with hallucinations and delusions associated with Parkinson's disease psychosis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include peripheral edema and confusional state (≥5% and twice the rate of placebo)..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Pimavanserin is indicated for the treatment of hallucinations and delusions associated with Parkinson's disease psychosis.
Dosage
- General Dosing Information
The recommended dose of Pimavanserin is 34 mg, taken orally as two 17 mg strength tablets once daily, without titration.
Pimavanserin can be taken with or without food.
- Dosage Modifications for Concomitant Use with CYP3A4 Inhibitors and Inducers
- Coadministration with Strong CYP3A4 Inhibitors
The recommended dose of Pimavanserin when coadministered with strong CYP3A4 inhibitors (e.g., ketoconazole) is 17 mg, taken orally as one tablet once daily.
- Coadministration with Strong CYP3A4 Inducers
Monitor patients for reduced efficacy if Pimavanserin is used concomitantly with strong CYP3A4 inducers; an increase in Pimavanserin dosage may be needed.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pimavanserin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pimavanserin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pimavanserin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pimavanserin in pediatric patients.
Contraindications
None
Warnings
|
INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete Boxed Warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Pimavanserin is not approved for the treatment of patients with dementia-related psychosis unrelated to the hallucinations and delusions associated with Parkinson's disease psychosis.
|
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Antipsychotic drugs increase the all-cause risk of death in elderly patients with dementia-related psychosis. Analyses of 17 dementia-related psychosis placebo-controlled trials (modal duration of 10 weeks and largely in patients taking atypical antipsychotic drugs) revealed a risk of death in the drug-treated patients of between 1.6- to 1.7-times that in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in placebo-treated patients.
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Pimavanserin is not approved for the treatment of patients with dementia-related psychosis unrelated to the hallucinations and delusions associated with Parkinson's disease psychosis.
QT Interval Prolongation
Pimavanserin prolongs the QT interval. The use of Pimavanserin should be avoided in patients with known QT prolongation or in combination with other drugs known to prolong QT interval including Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class 3 antiarrhythmics (e.g., amiodarone, sotalol), certain antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), and certain antibiotics (e.g., gatifloxacin, moxifloxacin). Pimavanserin should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and the presence of congenital prolongation of the QT interval.
Adverse Reactions
Clinical Trials Experience
The following serious adverse reactions are discussed elsewhere in the labeling:
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis
- QT Interval Prolongation
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The clinical trial database for Pimavanserin consists of over 1200 subjects and patients exposed to one or more doses of Pimavanserin. Of these, 616 were patients with hallucinations and delusions associated with Parkinson's disease psychosis (PDP). In the placebo-controlled setting, the majority of experience in patients comes from studies evaluating once-daily Pimavanserin doses of 34 mg (N=202) compared to placebo (N=231) for up to 6 weeks. In the controlled trial setting, the study population was approximately 64% male and 91% Caucasian, and the mean age was about 71 years at study entry. Additional clinical trial experience in patients with hallucinations and delusions associated with PDP comes from two open-label, safety extension studies (total N=497). The majority of patients receiving long-term treatment received 34 mg once-daily (N=459). Over 300 patients have been treated for more than 6 months; over 270 have been treated for at least 12 months; and over 150 have been treated for at least 24 months.
The following adverse reactions are based on the 6-week, placebo-controlled studies in which Pimavanserin was administered once daily to patients with hallucinations and delusions associated with PDP.
Common Adverse Reactions (incidence ≥5% and at least twice the rate of placebo): peripheral edema (7% Pimavanserin 34 mg vs. 2% placebo) and confusional state (6% Pimavanserin 34 mg vs. 3% placebo).
Adverse Reactions Leading to Discontinuation of Treatment
A total of 8% (16/202) of Pimavanserin 34 mg-treated patients and 4% (10/231) of placebo-treated patients discontinued because of adverse reactions. The adverse reactions that occurred in more than one patient and with an incidence at least twice that of placebo were hallucination (2% Pimavanserin vs. <1% placebo), urinary tract infection (1% Pimavanserin vs. <1% placebo), and fatigue (1% Pimavanserin vs. 0% placebo).
Adverse reactions that occurred in 6-week, placebo-controlled studies and that were reported at an incidence of ≥2% and >placebo are presented in Table 1.
- Table 1 Adverse Reactions in Placebo-Controlled Studies of 6-Week Treatment Duration and Reported in ≥2% and >Placebo

Adverse Reactions in Demographic Subgroups
Examination of population subgroups in the 6-week, placebo-controlled studies did not reveal any differences in safety on the basis of age (≤75 vs. >75 years) or sex. Because the study population was predominantly Caucasian (91%; consistent with reported demographics for PD/PDP), racial or ethnic differences in the safety profile of Pimavanserin could not be assessed. In addition, in the 6-week, placebo-controlled studies, no clinically relevant differences in the incidence of adverse reactions were observed among those with a Mini-Mental State Examination (MMSE) score at entry of <25 versus those with scores ≥25.
Postmarketing Experience
There is limited information regarding Pimavanserin Postmarketing Experience in the drug label.
Drug Interactions
Drugs Having Clinically Important Interactions with Pimavanserin
- Table 2 Clinically Important Drug Interactions with Pimavanserin

Drugs Having No Clinically Important Interactions with Pimavanserin
Based on pharmacokinetic studies, no dosage adjustment of carbidopa/levodopa is required when administered concomitantly with Pimavanserin.
Use in Specific Populations
Pregnancy
- Risk Summary
There are no data on Pimavanserin use in pregnant women that would allow assessment of the drug-associated risk of major congenital malformations or miscarriage. In animal reproduction studies, no adverse developmental effects were seen when Pimavanserin was administered orally to rats or rabbits during the period of organogenesis at doses up to 10- or 12-times the maximum recommended human dose (MRHD) of 34 mg/day, respectively. Administration of Pimavanserinto pregnant rats during pregnancy and lactation resulted in maternal toxicity and lower pup survival and body weight at doses which are 2-times the MRHD of 34 mg/day.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
- Data
- Animal Data
Pimavanserin was not teratogenic in pregnant rats when administered during the period of organogenesis at oral doses of 0.9, 8.5, and 51 mg/kg/day, which are 0.2- and 10-times the maximum recommended human dose (MRHD) of 34 mg/day based on AUC at mid and high doses, respectively. Maternal toxicity included reduction in body weight and food consumption at the highest dose.
Administration of Pimavanserin to pregnant rats during pregnancy and lactation at oral doses of 8.5, 26, and 51 mg/kg/day, which are 0.14- to 14-times the MRHD of 34 mg/day based on AUC, caused maternal toxicity, including mortality, clinical signs including dehydration, hunched posture, and rales, and decreases in body weight, and/or food consumption at doses ≥26 mg/kg/day (2-times the MRHD based on AUC). At these maternally toxic doses there was a decrease in pup survival, reduced litter size, and reduced pup weights, and food consumption. Pimavanserin had no effect on sexual maturation, neurobehavioral function including learning and memory, or reproductive function in the first generation pups up to 14-times the MRHD of 34 mg/day based on AUC.
Pimavanserin was not teratogenic in pregnant rabbits during the period of organogenesis at oral doses of 4.3, 43, and 85 mg/kg/day, which are 0.2- to 12-times the MRHD of 34 mg/day based on AUC. Maternal toxicity, including mortality, clinical signs of dyspnea and rales, decreases in body weight and/or food consumption, and abortions occurred at doses 12-times the MRHD of 34 mg/day based on AUC.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pimavanserin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pimavanserin during labor and delivery.
Nursing Mothers
There is no information regarding the presence of Pimavanserin in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Pimavanserin and any potential adverse effects on the breastfed infant from Pimavanserin or from the underlying maternal condition.
Pediatric Use
Safety and effectiveness of Pimavanserin have not been established in pediatric patients.
Geriatic Use
No dose adjustment is required for elderly patients.
Parkinson's disease is a disorder occurring primarily in individuals over 55 years of age. The mean age of patients enrolled in the 6-week clinical studies with Pimavanserin was 71 years, with 49% 65-75 years old and 31% >75 years old. In the pooled population of patients enrolled in 6-week, placebo-controlled studies (N=614), 27% had MMSE scores from 21 to 24 compared to 73% with scores ≥25. No clinically meaningful differences in safety or effectiveness were noted between these two groups.
Gender
There is no FDA guidance on the use of Pimavanserin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pimavanserin with respect to specific racial populations.
Renal Impairment
No dosage adjustment for Pimavanserin is needed in patients with mild to moderate (CrCL ≥30 mL/min, Cockcroft-Gault) renal impairment.
Use of Pimavanserin is not recommended in patients with severe renal impairment (CrCL <30 mL/min, Cockcroft-Gault). Pimavanserin has not been evaluated in this patient population.
Hepatic Impairment
Use of Pimavanserin is not recommended in patients with hepatic impairment. Pimavanserin has not been evaluated in this patient population.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pimavanserin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pimavanserin in patients who are immunocompromised.
Administration and Monitoring
Administration
The recommended dose of Pimavanserin is 34 mg, taken orally as two 17 mg strength tablets once daily, without titration.
Pimavanserin can be taken with or without food.
Monitoring
There is limited information regarding Pimavanserin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Pimavanserin and IV administrations.
Overdosage
Human Experience The pre-marketing clinical trials involving Pimavanserin in approximately 1200 subjects and patients do not provide information regarding symptoms with overdose. In healthy subject studies, dose-limiting nausea and vomiting were observed.
Management of Overdose There are no known specific antidotes for Pimavanserin. In managing overdose, cardiovascular monitoring should commence immediately and should include continuous ECG monitoring to detect possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide, and quinidine should not be used, as they have the potential for QT-prolonging effects that might be additive to those of Pimavanserin. Consider the long plasma half-life of Pimavanserin (about 57 hours) and the possibility of multiple drug involvement. Consult a Certified Poison Control Center (1-800-222-1222) for up-to-date guidance and advice.
Pharmacology
Mechanism of Action
The mechanism of action of Pimavanserin in the treatment of hallucinations and delusions associated with Parkinson's disease psychosis is unknown. However, the effect of Pimavanserin could be mediated through a combination of inverse agonist and antagonist activity at serotonin 5-HT2A receptors and to a lesser extent at serotonin 5-HT2C receptors.
Structure
There is limited information regarding Pimavanserin Structure in the drug label.
Pharmacodynamics
In vitro, Pimavanserin acts as an inverse agonist and antagonist at serotonin 5-HT2A receptors with high binding affinity (Ki value 0.087 nM) and at serotonin 5-HT2C receptors with lower binding affinity (Ki value 0.44 nM). Pimavanserin shows low binding to sigma 1 receptors (Ki value 120 nM) and has no appreciable affinity (Ki value >300 nM), to serotonin 5-HT2B, dopaminergic (including D2), muscarinic, histaminergic, or adrenergic receptors, or to calcium channels.
Cardiac Electrophysiology
The effect of Pimavanserin on the QTc interval was evaluated in a randomized placebo- and positive-controlled double-blind, multiple-dose parallel thorough QTc study in 252 healthy subjects. A central tendency analysis of the QTc data at steady-state demonstrated that the maximum mean change from baseline (upper bound of the two-sided 90% CI) was 13.5 (16.6) msec at a dose of twice the therapeutic dose. A pharmacokinetic/ pharmacodynamic analysis with Pimavanserin suggested a concentration-dependent QTc interval prolongation in the therapeutic range.
In the 6-week, placebo-controlled effectiveness studies, mean increases in QTc interval of ~5-8 msec were observed in patients receiving once-daily doses of Pimavanserin 34 mg. These data are consistent with the profile observed in a thorough QT study in healthy subjects. Sporadic QTcF values ≥500 msec and change from baseline values ≥60 msec were observed in subjects treated with Pimavanserin 34 mg; although the incidence was generally similar for Pimavanserin and placebo groups. There were no reports of torsade de pointes or any differences from placebo in the incidence of other adverse reactions associated with delayed ventricular repolarization in studies of Pimavanserin, including those patients with hallucinations and delusions associated with PDP.
Pharmacokinetics
Pimavanserin demonstrates dose-proportional pharmacokinetics after single oral doses from 17 to 255 mg (0.5- to 7.5-times the recommended dosage). The pharmacokinetics of Pimavanserin are similar in both the study population and healthy subjects. The mean plasma half-lives for Pimavanserin and the active metabolite (N-desmethylated metabolite) are approximately 57 hours and 200 hours, respectively.
Absorption
The median Tmax of Pimavanserin was 6 (range 4-24) hours and was generally unaffected by dose. The bioavailability of Pimavanserin oral tablet and Pimavanserin solution was essentially identical. The formation of the major circulating N-desmethylated metabolite AC-279 (active) from Pimavanserin occurs with a median Tmax of 6 hours.
Ingestion of a high-fat meal had no significant effect on rate (Cmax) and extent (AUC) of Pimavanserin exposure. Cmax decreased by about 9% while AUC increased by about 8% with a high-fat meal.
Distribution
Pimavanserin is highly protein bound (~95%) in human plasma. Protein binding appeared to be dose-independent and did not change significantly over dosing time from Day 1 to Day 14. Following administration of a single dose of Pimavanserin (34 mg), the mean (SD) apparent volume of distribution was 2173 (307) L.
Elimination
- Metabolism
Pimavanserin is predominantly metabolized by CYP3A4 and CYP3A5 and to a lesser extent by CYP2J2, CYP2D6, and various other CYP and FMO enzymes. CYP3A4 is the major enzyme responsible for the formation of its major active metabolite (AC-279). Pimavanserin does not cause clinically significant CYP inhibition or induction of CYP3A4. Based on in vitro data, Pimavanserin is not an irreversible inhibitor of any of the major hepatic and intestinal human CYP enzymes involved in drug metabolism (CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A4).
Based on in vitro studies, transporters play no significant role in the disposition of Pimavanserin.
AC-279 is neither a reversible or irreversible (metabolism-dependent) inhibitor of any of the major hepatic and intestinal human CYP enzymes involved in drug metabolism (CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A4). AC-279 does not cause clinically significant CYP3A induction and is not predicted to cause induction of any other CYP enzymes involved in drug metabolism.
- Excretion
Approximately 0.55% of the 34 mg oral dose of 14C-Pimavanserin was eliminated as unchanged drug in urine and 1.53% was eliminated in feces after 10 days.
Less than 1% of the administered dose of Pimavanserin and its active metabolite AC-279 were recovered in urine.
Specific Populations
Population PK analysis indicated that exposure of Pimavanserin in patients with mild to moderate renal impairment was similar to exposure in patients with normal renal function. Age, sex, ethnicity, and weight do not have clinically relevant effect on the pharmacokinetics of Pimavanserin.
Pimavanserin has not been studied in patients with severe renal impairment or mild to severe hepatic impairment.
Drug Interaction Studies
CYP3A4 Inhibitor: ketoconazole, a strong inhibitor of CYP3A4, increased Pimavanserin Cmax by 1.5-fold and AUC by 3-fold.
The effect of Pimavanserin on other drugs is shown in Figure 1.
- Figure 1 The Effects of Pimavanserin on the Pharmacokinetics of Other Drugs

NUPLAZID: Pimavanserin's Brand name
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no increase in the incidence of tumors following daily oral administration of Pimavanserin to mice or rats for 2 years. Mice were administered Pimavanserin at oral doses of 2.6, 6, and 13 (males)/8.5, 21, and 43 mg/kg/day (females) which are 0.01- to 1- (males)/0.5- to 7- (females) times the MRHD of 34 mg/day based on AUC. Rats were administered Pimavanserin at oral doses of 2.6, 8.5, and 26 (males)/4.3, 13, and 43 mg/kg/day (females) which are 0.01- to 4- (males)/0.04- to 16- (females) times the MRHD of 34 mg/day based on AUC.
Pimavanserin was not mutagenic in the in vitro Ames reverse mutation test, or in the in vitro mouse lymphoma assay, and was not clastogenic in the in vivo mouse bone marrow micronucleus assay.
- Impairment of Fertility
Pimavanserin was administered orally to male and female rats before mating, through mating, and up to Day 7 of gestation at doses of 8.5, 51, and 77 mg/kg/day, which are approximately 2-, 15-, and 22-times the maximum recommended human dose (MRHD) of 34 mg/day based on mg/m2, respectively. Pimavanserin had no effect on fertility or reproductive performance in male and female rats at doses up to 22-times the MRHD of 34 mg based on mg/m2. Changes in uterine parameters (decreases in the number of corpora lutea, number of implants, viable implants, and increases in pre-implantation loss, early resorptions and post-implantation loss) occurred at the highest dose which was also a maternally toxic dose. Changes in sperm parameters (decreased density and motility) and microscopic findings of cytoplasmic vacuolation in the epididymis occurred at doses approximately 15-times the MRHD of 34 mg/day based on mg/m2.
Animal Toxicology and/or Pharmacology
Phospholipidosis (foamy macrophages and/or cytoplasmic vacuolation) was observed in multiple tissues and organs of mice, rats, and monkeys as early as 14 days following oral daily administration of Pimavanserin. The most severely affected organs were the lungs and kidneys. The occurrence of phospholipidosis was both dose- and duration-dependent. Diffuse phospholipidosis with focal/multifocal chronic inflammation was observed in the lungs of rats treated for ≥3 months at doses ≥10-times the maximum recommended human dose (MRHD) of 34 mg/day based on AUC. As a result of chronic inflammation, inflammatory lung fibrosis was observed in rats treated for 3 and 6 months at doses ≥18-times the MRHD of 34 mg/day based on AUC. The findings in the lungs correlated with increased lung weights (up to 3-times those of controls) and respiratory-related clinical signs including rales, labored breathing, and gasping. Phospholipidosis in lungs of rats caused mortality at doses ≥16-times the MRHD of 34 mg/day based on AUC. The estimated No Observed Effect Level (NOEL) for chronic lung inflammation in rats is 5-fold the MRHD of 34 mg/day based on AUC. Phospholipidosis was associated with increased kidney weights and tubular degeneration in rats at doses ≥10-times the MRHD of 34 mg/day based on AUC. The relevance of these findings to human risk is not known.
Clinical Studies
The efficacy of Pimavanserin 34 mg as a treatment of hallucinations and delusions associated with Parkinson's disease psychosis was demonstrated in a 6-week, randomized, placebo-controlled, parallel-group study. In this outpatient study, 199 patients were randomized in a 1:1 ratio to Pimavanserin 34 mg or placebo once daily. Study patients (male or female and aged 40 years or older) had a diagnosis of Parkinson's disease (PD) established at least 1 year prior to study entry and had psychotic symptoms (hallucinations and/or delusions) that started after the PD diagnosis and that were severe and frequent enough to warrant treatment with an antipsychotic. At entry, patients were required to have a Mini-Mental State Examination (MMSE) score ≥21 and to be able to self-report symptoms. The majority of patients were on PD medications at entry; these medications were required to be stable for at least 30 days prior to study start and throughout the study period.
The PD-adapted Scale for the Assessment of Positive Symptoms (SAPS-PD) was used to evaluate the efficacy of Pimavanserin 34 mg. SAPS-PD is a 9-item scale adapted for PD from the Hallucinations and Delusions domains of the SAPS. Each item is scored on a scale of 0-5, with 0 being none and 5 representing severe and frequent symptoms. Therefore, the SAPS-PD total score can range from 0 to 45 with higher scores reflecting greater severity of illness. A negative change in score indicates improvement. Primary efficacy was evaluated based on change from baseline to Week 6 in SAPS-PD total score.
As shown in Table 3, Figure 2, and Figure 3, Pimavanserin 34 mg (n=95) was statistically significantly superior to placebo (n=90) in decreasing the frequency and/or severity of hallucinations and delusions in patients with PDP as measured by central, independent, and blinded raters using the SAPS-PD scale. An effect was seen on both the hallucinations and delusions components of the SAPS-PD.
- Table 3 Primary Efficacy Analysis Result Based on SAPS-PD (N=185)

NUPLAZID: Pimavanserin's Brand name
The effect of Pimavanserin on SAPS-PD improved through the six-week trial period, as shown in Figure 2.
- Figure 2 SAPS-PD Change from Baseline through 6 Weeks Total Study Treatment

NUPLAZID: Pimavanserin's Brand name
- Figure 3 Proportion of Patients with SAPS-PD Score Improvement at the End of Week 6 (N=185)

NUPLAZID: Pimavanserin's Brand name
Motor Function in Patients with Hallucinations and Delusions Associated with Parkinson's Disease Psychosis
Pimavanserin 34 mg did not show an effect compared to placebo on motor function, as measured using the Unified Parkinson's Disease Rating Scale Parts II and III (UPDRS Parts II+III) (Figure 4). A negative change in score indicates improvement. The UPDRS Parts II+III was used to assess the patient's Parkinson's disease state during the 6-week double-blind treatment period. The UPDRS score was calculated as the sum of the 40 items from activities of daily living and motor examination, with a range of 0 to 160.
- Figure 4 Motor Function Change from Baseline to Week 6 in UPDRS Parts II+III (LSM - SE)

NUPLAZID: Pimavanserin's Brand name
How Supplied
Pimavanserin tablets are available as:
17 mg Tablet:
White to off-white, round, immediate-release, film-coated tablet debossed with "P" on one side and "17" on the reverse. . Each tablet contains 20 mg of pimavanserin tartrate, which is equivalent to 17 mg of Pimavanserin free base. Inactive ingredients include pregelatinized starch, magnesium stearate, and microcrystalline cellulose. Additionally, the following inactive ingredients are present as components of the film coat: hypromellose, talc, titanium dioxide, polyethylene glycol, and saccharin sodium.
Bottle of 60: NDC 63090-170-60
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F).
Images
Drug Images
{{#ask: Page Name::Pimavanserin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Pimavanserin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise patients to inform their healthcare providers if there are any changes to their current prescription or over-the-counter medications, since there is a potential for drug interactions.
Precautions with Alcohol
Alcohol-Pimavanserin interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
NUPLAZID™
Look-Alike Drug Names
There is limited information regarding Pimavanserin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 Friedman, JH (October 2013). "Pimavanserin for the treatment of Parkinson's disease psychosis". Expert Opinion on Pharmacotherapy. 14 (14): 1969–1975. doi:10.1517/14656566.2013.819345. PMID 24016069.
- ↑ "Nuplazid (pimavanserin) Tablets, for Oral Use. U.S. Full Prescribing Information" (PDF). ACADIA Pharmaceuticals Inc. Retrieved 1 May 2016.