Phenylephrine (oral): Difference between revisions
Adeel Jamil (talk | contribs) No edit summary |
Adeel Jamil (talk | contribs) No edit summary |
||
| Line 111: | Line 111: | ||
* Each mL contains: Phenylephrine Hydrochloride 10 mg; Sodium Chloride 3.5 mg; Sodium Citrate Dihydrate 4 mg; Citric Acid Monohydrate 1 mg; Sodium Metabisulfite 2 mg; Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid if necessary. pH 3.0-6.5. | * Each mL contains: Phenylephrine Hydrochloride 10 mg; Sodium Chloride 3.5 mg; Sodium Citrate Dihydrate 4 mg; Citric Acid Monohydrate 1 mg; Sodium Metabisulfite 2 mg; Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid if necessary. pH 3.0-6.5. | ||
|storage=* Store at room temperature (59°-86°F) | |storage=* Store at room temperature (59°-86°F) | ||
|packLabel=[[File: | |packLabel=[[File:Phenylephrine oral drug lable.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
|alcohol=Alcohol-Phenylephrine (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Phenylephrine (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* PHENYLEPHRINE HYDROCHLORIDE®<ref>{{Cite web | title = PHENYLEPHRINE HYDROCHLORIDE phenylephrine hydrochloride injection | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1a7ab4b-0afe-4463-a039-5be0323cf2f7 }}</ref> | |brandNames=* PHENYLEPHRINE HYDROCHLORIDE®<ref>{{Cite web | title = PHENYLEPHRINE HYDROCHLORIDE phenylephrine hydrochloride injection | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1a7ab4b-0afe-4463-a039-5be0323cf2f7 }}</ref> | ||
}} | }} | ||
Revision as of 03:23, 26 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Phenylephrine (oral) is an alpha-1 adrenergic receptor agonist that is FDA approved for the treatment of temporarily relieves sinus congestion and pressure, temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies. Common adverse reactions include nausea, vomiting, headache, and nervousness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Temporarily relieves sinus congestion and pressure.
- Temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies.
Dosing Information
- take every 4 hours
- do not take more than 6 doses in 24 hours
- adults and children 12 years of age and over: 1 tablet
- children under 12 years of age: ask a doctor
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Phenylephrine (oral) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Phenylephrine (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Phenylephrine (oral) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Phenylephrine (oral) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Phenylephrine (oral) in pediatric patients.
Contraindications
There is limited information regarding Phenylephrine (oral) Contraindications in the drug label.
Warnings
- Do not use if you are now taking a prescription monoamine exidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have:
- heart disease
- high blood pressure
- diabetes
- trouble urinating due to an enlarge prostate gland
- thyroid disease
- When using this product, do not use more than directed.
- Stop use and ask a doctor if:
- you get nervous
- dizzy or
- sleepless
- symptoms do not improve within 7 days or are accompanied by fever
- If pregnant or breast-feeding, ask a health professional before use.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Phenylephrine (oral) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Phenylephrine (oral) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Phenylephrine (oral) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Phenylephrine (oral) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Phenylephrine (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Phenylephrine (oral) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Phenylephrine (oral) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Phenylephrine (oral) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Phenylephrine (oral) in geriatric settings.
Gender
There is no FDA guidance on the use of Phenylephrine (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Phenylephrine (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Phenylephrine (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Phenylephrine (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Phenylephrine (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Phenylephrine (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Phenylephrine (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Phenylephrine (oral) and IV administrations.
Overdosage
There is limited information regarding Phenylephrine (oral) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Phenylephrine hydrochloride produces vasoconstriction that lasts longer than that of epinephrine and ephedrine. Responses are more sustained than those to epinephrine, lasting 20 minutes after intravenous and as long as 50 minutes after subcutaneous injection. Its action on the heart contrasts sharply with that of epinephrine and ephedrine, in that it slows the heart rate and increases the stroke output, producing no disturbance in the rhythm of the pulse.
- Phenylephrine is a powerful postsynaptic alpha-receptor stimulant with little effect on the beta receptors of the heart. In therapeutic doses, it produces little if any stimulation of either the spinal cord or cerebrum. A singular advantage of this drug is the fact that repeated injections produce comparable effects.
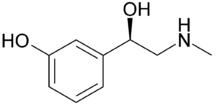
Structure
- Phenylephrine hydrochloride is a vasoconstrictor and pressor drug chemically related to epinephrine and ephedrine. Phenylephrine hydrochloride is a synthetic sympathomimetic agent in sterile form for parenteral injection. Chemically, phenylephrine hydrochloride is (-)-m-Hydroxy-α-[(methylamino)methyl]benzyl alcohol hydrochloride, and has the following structural formula:
- Each mL contains: Phenylephrine Hydrochloride 10 mg; Sodium Chloride 3.5 mg; Sodium Citrate Dihydrate 4 mg; Citric Acid Monohydrate 1 mg; Sodium Metabisulfite 2 mg; Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid if necessary. pH 3.0-6.5.
Pharmacodynamics
There is limited information regarding Phenylephrine (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Phenylephrine (oral) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Phenylephrine (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Phenylephrine (oral) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Phenylephrine (oral) How Supplied in the drug label.
Storage
- Store at room temperature (59°-86°F)
Images
Drug Images
{{#ask: Page Name::Phenylephrine (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
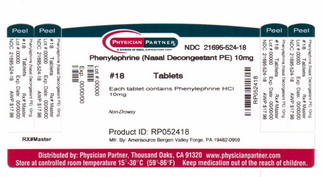
{{#ask: Label Page::Phenylephrine (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Phenylephrine (oral) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Phenylephrine (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PHENYLEPHRINE HYDROCHLORIDE®[1]
Look-Alike Drug Names
There is limited information regarding Phenylephrine (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.