Phenylbutazone
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H20N2O2 |
| Molar mass | 308.374 g/mol |
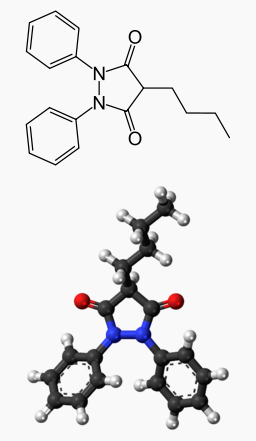
Phenylbutazone, often referred to as bute, is a crystalline substance having the structure shown at right.
- Structural name: 4-butyl-1,2-diphenyl-3,5-pyrazolidinedione
- Chemical formula: C19H20N2O2
Oxyphenbutazone, the major metabolite of phenylbutazone, differs only in the para location of one of its phenyl groups, where a hydrogen atom is replaced by a hydroxyl group (making it 4-butyl-1-(4-hydroxyphenyl)-2-phenyl-3,5-pyrazolidinedione).
Despite its name, phenylbutazone is chemically unrelated to the class of chemicals known as benzones (common examples include oxybenzone, dioxybenzone, avobenzone, and sulisobenzone), which are used as active ingredients in sunscreen formulations for protection against UVB rays.
Phenylbutazone is used as a non-steroidal anti-inflammatory drug (NSAID) for the treatment of chronic pain, including the symptoms of arthritis. Its use is limited by such severe side effects as suppression of white blood cell production and aplastic anemia.
The International Agency for Research on Cancer (IARC) places it in Group 3; i.e., "not classifiable as to its carcinogenicity to humans."
Uses of phenylbutazone in horses
Phenylbutazone is an NSAID commonly used in horses for the following purposes:
- Analgesia: Pain relief from infections and musculoskeletal disorders including sprains, overuse injuries, tendinitis, arthralgias, arthritis, and laminitis. Like other NSAIDs, acts directly on musculoskeletal tissue to control inflammation, thereby reducing secondary inflammatory damage, alleviating pain, and restoring range of motion. Does not cure musculoskeletal ailments or work well on colic pain.
- Antipyresis: Reduction of fevers. Antipyretic qualities may mask other symptoms; therefore, should not be administered for this purpose unless a veterinarian has concluded that the horse would not be able to eat or drink without its use or that the fever might hinder the horse's recovery.
Dosage and administration
Phenylbutazone may be administered orally (via paste, powder or feed-in) or intravenously. It should not be given intramuscularly or injected in any place other than a vein, as it can cause tissue damage. Tissue damage and edema may also occur if the drug is injected repetitively into the same vein.
The maximum oral dose recommended by manufacturers is 2 to 4 grams per 1000 pounds of body weight (4 to 9 mg/kg) per day. Manufacturers recommend that the dose be divided equally and given every 8 hours for maximum results, although most horse owners give it every 12 to 24 hours for convenience, usually giving 1 to 2 grams in the morning and at night.
Intravenously, the maximum daily dose recommended by manufacturers is 1 to 2 grams per 1000 pounds (2 to 4.5 mg/kg). The maximum dose is usually given when the course of treatment is initiated, with the dosage subsequently being titrated down.
Phenylbutazone should be administered only under the advice of a veterinarian.
Side effects and disadvantages of phenylbutazone
Side effects of phenylbutazone are similar to that of other NSAIDs. Overdose or prolonged use can cause gastrointestinal ulcers, blood dyscrasia, kidney damage, oral lesions, and internal hemorrhage, especially pronounced in young, ill, or stressed horses. Effects of gastrointestinal damage include edema of the legs and belly secondary to leakage of blood proteins into the intestines, resulting in decreased appetite, excessive thirst, weight loss, weakness, and in advanced stages, kidney failure and death.
Phenylbutazone should not be used in combination with blood thinners (e.g., Coumadin), as it amplifies the anticoagulant effects of these drugs; with other NSAIDs (all NSAIDs are additive); or in horses with known kidney or liver problems.
Periodic blood tests are recommended when using phenylbutazone as Agranulocytosis can occur. Periodically testing the blood may catch this issue before it is too late.
Phenylbutazone should be used cautiously in pregnant or nursing mares, as it may be toxic to the embryo and can be transferred via the umbilical cord and by milk.
High doses of phenylbutazone may be considered a rules violation under some equestrian organizations, as the drug may remain in the bloodstream four to five days after administration.
In humans, Phenylbutazone is very dangerous, as it can cause aplastic anemia. The medicine should be given in a paste form to avoid contact with the medicine. Never breathe powder from crushing tablets.
History of phenylbutazone in racing
In the 1968 Kentucky Derby, Dancer's Image, the winner of the race, was disqualified after traces of phenylbutazone were discovered in a post-race urinalysis. Owned by prominent Massachusetts businessman Peter Fuller and jockeyed by Bobby Ussery, Dancer's Image remains the only horse in history to win the Kentucky Derby and then be disqualified. Phenylbutazone was legal on most tracks around the country in 1968 but had not yet been approved by Churchill Downs. Also, in the weeks prior to the race Peter Fuller had given his purse to Coretta Scott King, the widow of slain Civil Rights activist Martin Luther King Jr., an act that brought him both praise from Civil Rights supporters and criticism from racists. Martin Luther King Jr. had helped plan a sit-in on the Kentucky Derby the year before but did not allow it to proceed. [1]
Controversy and speculation still surrounds the incident. Although after many appeals, Forward Pass was named the winner, the Kentucky Derby official website lists both Dancer's Image and Forward Pass as the winner. The website's race video commentary states that on the winner's plaque at Churchill Downs both Dancer's Image and Forward Pass are listed as the 1968 winner of the Kentucky Derby.[2]
Other significance
- Oxyphenbutazone is the highest (known) scoring word in Scrabble.[3]
References
- ↑ "Sports: Dancer's tainted image". Retrieved 2007-10-07.
- ↑ "Kentucky Derby 132". Retrieved 2007-10-07. Text " 2006 " ignored (help); Text " Derby History " ignored (help); Text " Years " ignored (help); Text " 1968 " ignored (help); Text " Derby Charts " ignored (help)
- ↑ Miller, Jeff. "A Collection of Word Oddities and Trivia". Unknown parameter
|accessyear=ignored (|access-date=suggested) (help); Unknown parameter|accessmonthday=ignored (help)
External links
- IARC monograph on carcinogenicity of phenylbutazone
- Phenylbutazone For Veterinary Use
- Resource on Equine Joint Health
- Pages with script errors
- Pages with citations using unnamed parameters
- Pages with citations using unsupported parameters
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Non-steroidal anti-inflammatory drugs
- Equine medications
- Pyrazolones
- Drugs