Perinatal infection
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
perinatal is Referring to the period of time surrounding an infant's birth, from the last two months of pregnancy through the first 28 days of life
Historical Perspective
In the 1970s investigators at Emory University and the Centers for Disease Control and Prevention (CDC) coined the term TORCH, an acronym underscoring Toxoplasma gondii, rubella virus, cytomegalovirus, and herpesviruses as important, potential causes of congenital infection (Nahmias, 1974). The TORCH concept emphasized that these agents can produce similar clinical manifestations in infected infants. Although congenital rubella virus syndrome has since disappeared in countries with compulsory immunization against this virus (CDC, 2005; WHO, 2017), the TORCH agents, as well as more recently recognized pathogens, such as lymphocytic choriomeningitis virus and Zika virus, remain major causes of long-term neurodevelopmental disabilities among children throughout the world (Bale, 2009).[1]
Classification
a perinatal infection: is vertically transmitted infection ,which starts at gestational ages between 22[2] and 28 weeks[3] (with regional variations in the definition) and ending seven completed days after birth.[2]
Heading text
Pathophysiology
In the spectrum of optimal virulence, vertical transmission tends to evolve benign symbiosis, so is a critical concept for evolutionary medicine. Because a pathogen's ability to pass from mother to chd depends significantly on the hosts' ability to reproduce, pathogens' transmissibility tends to be inversely related to their virulence. In other words, as pathogens become more harmful to, and thus decrease the reproduction rate of, their host organism, they are less likely to be passed on to the hosts' offspring, since they will have fewer offspring.[4]
Although HIV is sometimes transmitted through perinatal transmission, its virulence can be accounted for because its primary mode of transmission is not vertical. Moreover, medicine has further decreased the frequency of vertical transmission of HIV. The incidence of perinatal HIV cases in the United States has declined as a result of the implementation of recommendations on HIV counselling and voluntary testing practices and the use of zidovudine therapy by providers to reduce perinatal HIV transmission.[5]
The price paid in the evolution of symbiosis is, however, great: for many generations, almost all cases of vertical transmission continue to be pathological—in particular if any other routes of transmission exist. Many generations of random mutation and selection are needed to evolve symbiosis. During this time, the vast majority of vertical transmission cases exhibit the initial virulence.[citation needed]
In dual inheritance theory, vertical transmission refers to the passing of cultural traits from parents to children.[6]
Causes
perinatal infection may be caused TORCHES CLAP
- Toxoplasmosis
- Rubella
- Cytomegalovirus
- chylamedia
- Herpes simplex
- Hepatitis B& C
- Enterovirus
- Syphilis
- group B streptococcus
- Chicken box
- Lyme disease
- listeriosis
- AIDS
- Parvovirus B19
lastest addition:Zika virus
Differentiating [disease name] from other Diseases
- [Disease name] must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as:
- [Differential dx1]
- [Differential dx2]
- [Differential dx3]
Epidemiology and Demographics
- The prevalence of [perinatal infection ] depends on the causative agent of infection. For example, perinatal transmission of cytomegalovirus occurs in two to 24 out of every 1,000 live births. The rate of transmission of genital herpes during pregnancy is one to two out of every 2,000 pregnancies; the rate of transmission during childbirth changes to one out of every 2,000 to 5,000 live births. Perinatal transmission of group beta streptococcus causes neonatal infection in one to five out of every 1,000 live births, and rubella (German measles ), 0.02 out of every 1,000 live births. HIV is transmitted from untreated mother to child in 25 to 40 percent of cases, but in only 1 percent of cases if mother receives treatment and the infant receives prophylaxis.
Age
- Patients of all age groups may develop [ perinatal infection].
Gender
- [ perinatal infection ] affects boy and girls children equally.
Race
- There is no racial predilection for [ perinatal infection].
Risk Factors
- Common risk factors in the development of [Perinatal infection ] Page text.[7]are
| Fetal causes | maternal causes |
|---|---|
| Birth weight | chorioamnionitis |
| Ceseran delivary | Hypertension (pregestational and gestational including preeclampsia) |
| Multiple delivary | Diabetes (pregestational and gestational) |
| Fetal distress | |
| Meconium aspiration | |
| Patent ductus arteriosus |
Infant outcomes
| Infant outcome |
|---|
| Mechanical ventilation |
| Pneumothorax |
| Respiratory distress syndrome |
| Chronic lung disease |
| Necrotizing enterocolitis |
| Interventricular hemorrhage |
| Hypoxic - ischemic encephalopathy |
| Retinopathy of prematurity |
| Extracorporeal life support |
| In hospital death |
In addition, we evaluated combined grade 3 and grade 4 intraventricular hemorrhage and combined stages 3 through 5 ROP to align with common categorization of these more clinically important outcomes.
Natural History, Complications and Prognosis
- The majority of patients with [disease name] remain asymptomatic for [duration/years].
- Early clinical features include [manifestation 1], [manifestation 2], and [manifestation 3].
- If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
- Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
- Prognosis is generally [excellent/good/poor], and the [1/5/10year mortality/survival rate] of patients with [disease name] is approximately [#%].
The prognosis of a neonate who has contracted an infection perinatally depends on the specific infection.[8]Examples include the following:
- Chlamydia: Without treatment, the most serious consequences of chlamydial infection are related to complications of premature delivery. Treatment of the mother with antibiotics during the third trimester can prevent premature delivery and the transfer of the infection to the baby. Infants treated with antibiotics for eye infection or pneumonia generally recover.
- Cytomegalovirus: The chance for recovery after exposure to CMV is very good for both the mother and the infant. Exposure to CMV can be serious and even life threatening for mothers and infants whose immune systems are compromised, for example, those receiving chemotherapy or who have HIV/AIDS. Those infants who develop birth defects after CMV exposure may have serious, lifelong complications.
- Genital herpes: Once a woman or infant is infected, outbreaks of genital herpes sores can recur at any point during their lifetimes.
- Hepatitis B: Infants treated at birth with immune globulin and the series of vaccinations are protected from development of hepatitis B infection. Infants infected with hepatitis B develop a chronic, mild form of hepatitis and are at increased risk for developing liver disease.
- Human immunodeficiency virus (HIV): A combination of treatment with highly active antiretroviral therapy during pregnancy, zidovudine (AZT) during delivery, and AZT to the baby for six weeks after birth significantly reduces the chance that the infant will be infected with HIV from the mother.
- Human papillomavirus: Once infected with HPV, there is a lifelong risk of developing warts and an increased risk of some cancers.
- Rubella (German measles): Infants exposed to rubella virus in the uterus are at high risk for severe birth defects, including heart defects, blindness, and deafness.
- Streptococcus: Infection of the urinary tract or genital tract of pregnant women can cause premature birth. Infants infected with GBS can develop serious, life-threatening infections.
- Syphilis: Premature birth, birth defects, or the development of serious syphilis symptoms is likely to occur in untreated pregnant women
Diagnosis
Diagnostic Criteria
- The diagnosis of [perinatal diagnosis] [9]is made when
- Chlamydia can be diagnosed by taking a cotton swab sample of the cervix and vagina during the third trimester of the pregnancy. Chlamydial cell cultures take three to seven days to grow. DNA probes are available for more rapid diagnosis.
- Past or recent infection with cytomegalovirus (CMV) can be identified by documentation of seroconversion of a previously seronegative patient (the development of IgG antibodies to CMV in a patient who was previously negative for these antibodies) and CMV can be grown from body fluids.[10]
- Genital herpes is suspected with the outbreak of a particular kind of genital sore. The sore can be cultured and tested to confirm that HSV-2 is present.
- Hepatitis B can be identified through a blood test for the hepatitis B surface antigen (HBsAg) in pregnant women. The test is part of prenatal health programs.
- Human immunodeficiency virus (HIV) can be detected using a blood test and is part of most prenatal screening programs.
- Human papillomavirus (HPV) causes the growth of warts in the genital area. The wart tissue can be removed with a scalpel and tested to determine what type of HPV virus caused the infection.
- Pregnant women are usually tested for antibodies to rubella, which would indicate that they have been previously exposed to the virus and, therefore, would not develop infection during pregnancy if exposed.
- Group beta streptococcus (GBS) can be detected by a vaginal or rectal swab culture and sometimes from a urine culture. Blood tests can be used to confirm GBS infection in infants who exhibit symptoms.
- Pregnant women are usually tested for syphilis as part of the prenatal screening, generally with a blood test.
- ZIKA virus Methods for testing include both serologic and molecular tests. Laboratory tests in include ZIKV IgM, ZIKV NAT, and plaque reduction neutralization testing.[11]
History and Symptoms
If a developing fetus is infected by a TORCH agent, the outcome of the pregnancy may be miscarriage, stillbirth, delayed fetal growth and maturation (intrauterine growth retardation), or early delivery. In addition, newborns infected by any one of the TORCH agents may develop a spectrum of similar symptoms and findings. These may include
- listlessness (lethargy),
- fever,
- difficulties feeding,
- enlargement of the liver and spleen (hepatomegaly),
- and decreased levels of the oxygen-carrying pigment (hemoglobin) in the blood (anemia).
In addition, affected infants may develop
- areas of bleeding, resulting in reddish or purplish spots or areas of discoloration visible through the skin (petechia or purpura);
- yellowish discoloration of the skin, whites of the eyes, and mucous membranes (jaundice);
- inflammation of the middle and innermost layers of the eyes (chorioretinitis); and/or other symptoms and findings.
Each infectious agent may also cause additional abnormalities that may vary in degree and severity, depending upon the stage of fetal development at time of infection and/or other factors.
Following is a more specific description of the TORCH agents.
Toxoplasmosis is an infectious disease caused by the microscopic parasitic organism called Toxoplasma gondii. Classic triad of toxoplasmosis Chorioretinitis (a form of posterior uveitis) Diffuse intracranial calcifications Hydrocephalus
Rubella is a viral infection characterized by fever, upper respiratory infection, swelling of the lymph nodes, skin rash, and joint pain. Severely affected newborns and infants may have visual and/or hearing impairment, heart defects, calcium deposits in the brain, and/or other abnormalities.
Cytomegalovirus (CMV) Infection is a viral infection that may occur during pregnancy, after birth, or at any age. In severely affected newborns, associated symptoms and findings may include growth retardation, an abnormally small head (microcephaly), enlargement of the liver and spleen (hepatosplenomegaly), inflammation of the liver (hepatitis), low levels of the oxygen-carrying pigment in the blood due to premature destruction of red blood cells (hemolytic anemia), calcium deposits in the brain, and/or other abnormalities.
Neonatal Herpes is a rare disorder affecting newborns infected with the Herpes simplex virus (HSV). This disorder may vary from mild to severe. In most cases, the disorder is transmitted to an infant from an infected mother with active genital lesions at the time of delivery. In the event that a mother has a severe primary genital outbreak, it is possible that a mother may transmit the infection to the fetus. After delivery, direct contact with either genital or oral herpes sores may result in neonatal herpes. Severely affected newborns may develop fluid-filled blisters on the skin (cutaneous vesicles), lesions in the mouth area, inflammation of the mucous membrane lining the eyelids and whites of the eyes (conjunctivitis), abnormally diminished muscle tone, inflammation of the liver (hepatitis), difficulties breathing, and/or other symptoms and findings.
Parvovirus B19 Infection during pregnancy occurs in 1–5% of pregnancies. The virus can cause miscarriage, fetal anaemia, hydrops fetalis (abnormal accumulation of fluid in the fetal tissues), myocarditis, and/or intrauterine fetal death.
TEXTBOOKS Nelson Textbook of Pediatrics, 15th Ed.: Richard E. Behrman, Editor; W.B. Saunders Company, 1996. Pp. 518, 521. JOURNAL ARTICLES The Torch Syndrome, A Clinical Review. J. D. Fine et al.; J Amer Acad Dermatol (April 1985; 12(4)). Pp. 2477-78. Torch, A Literature Review and Implications for Practice. L. Haggerty; J Obstet Gynecol Nurs (March-April 1985; 14(2)). Pp. 124-29. Timely Diagnosis of Congenital Infections. J.K. Stamos et al.; Pediatr Clin North Am (Oct 1994; 41(5)). Pp. 1017-33. Torch Syndrome. R.E. Epps et al.; Semin Dermatol (Jun 1995; 14(2)). Pp. 179-86. Torch Congenital Infections. E. Domenech et al.; An Esp Pediatr (Jun 1997; Spec No 1). Pp. 58-62. Serologic and DNA-Based Testing for Congenital and Perinatal Infections. C.M. Litwin et al.; Pediatr Infect Dis J (Dec 1997; 16(12)). Pp. 1166-75. Current Use of the Torch Screen in the Diagnosis of Congenital Infection. A. Cullen et al.; J Infect (Mar 1998; 36(2)). Pp. 185-88. Torch Syndrome. Y. Hidaka et al.; Ryoikibetsu Shokogun Shirizu (1999; 25(Pt 3)). Pp. 85-88.
Syphilis Early congenital syphilis Hepatomegaly and jaundice Rhinorrhea with white or bloody nasal discharge Maculopapular rash on palms and soles Skeletal abnormalities (e.g., metaphyseal dystrophy, periostitis) Generalized lymphadenopathy (nontender)
Listeriosis Intrauterine transmission Increased risk of premature birth and spontaneous abortion Early-onset syndrome: granulomatosis infantiseptica Severe systemic infection characterized by disseminated abscesses (may develop in any organ system) Most common findings: respiratory distress and skin lesions Signs of meningitis may already develop. Transmission during birth or postnatally (via contact with the mother or contaminated environment)
enterovirus
Wide spectrum of clinical presentations,
from non-specific febrile illness to fatal multisystem disease, Fever, irritability,poor feeding, lethargy Maculopapular rash in 50% Respiratory symptoms in 50% Gastrointestinal symptoms in 20% Hepatitis in 50% May have myocarditis, meningoencephalitis
Physical Examination
| Finding(s) | Possible congenital infections |
|---|---|
| Intrauterine growth retardation | Rubella, cytomegalovirus (CMV), toxoplasmosis |
| Anemia with hydrops | Parvovirus B19, syphilis, CMV, toxoplasmosis |
| Bone lesions | Syphilis, rubella |
| Cerebral calcification |
|
| Congenital heart disease | Rubella |
| Hearing loss (commonly progressive) | Rubella, CMV, toxoplasmosis, syphilis |
| Hepatosplenomegaly | CMV, rubella, toxoplasmosis, HSV, syphilis, enterovirus, parvovirus B19 |
| Hydrocephalus | Toxoplasmosis, CMV, syphilis, possibly enterovirus |
| Hydrops, ascites, pleural effusions | Parvovirus B19, CMV, toxoplasmosis, syphilis |
| Jaundice with or without thrombocytopenia | CMV, toxoplasmosis, rubella, HSV, syphilis, enterovirus |
| Limb paralysis with atrophy and cicatrices | Varicella |
| Maculopapular exanthem | Syphilis, measles, rubella, enterovirus |
| Microcephaly | CMV, toxoplasmosis, rubella, varicella, HSV |
| Myocarditis/encephalomyocarditis | Echovirus, coxsackie B, other enterovirus |
| Ocular findings | CMV, toxoplasmosis, rubella, HSV, syphilis, enterovirus, parvovirus B19 |
| Progressive hepatic failure and clotting abnormalities | Echovirus, coxsackie B, other enterovirus, HSV, toxoplasmosis |
| Pseudoparalysis, pain | Syphilis |
| Vesicles | HSV, syphilis, varicella, enterovirus |
Laboratory Findings
- rubella may be diagnosed by detection of specific IgM, but virus detection is the technique of choice.
- VZV may be diagnosed by serological techniques in up to 71% of cases. Detection of virus in vesicle scrapings or swabs from the oropharynx is the technique of choice for neonatal HSV.
- enterovirus infections are best diagnosed by detection of viral RNA.
- HIV-1 may be diagnosed within 3 months of birth by testing serial blood samples with a combination of techniques. Maternal infection with HBV, HCV, HIV and HTLV1/11 may be diagnosed by serological techniques and genital PVs by detection of viral DNA. Chorionic villus samples, amniotic fluid and fetal blood may be obtained for prenatal diagnosis of infection.
- detection of virus in amniotic fluid is the technique of choice for prenatal diagnosis of CMV, insufficient data is currently available to determine whether it may be used for intrauterine rubella.
- The most reliable technique for diagnosis of fetal B19 infection is detection of viral DNA in fetal blood.
the use of TORCH screening should be discouraged.15566870
Electrocardiogram
There are no ECG findings associated with [disease name].
OR
An ECG may be helpful in the diagnosis of [disease name]. Findings on an ECG suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
X-ray
There are no x-ray findings associated with [disease name].
OR
An x-ray may be helpful in the diagnosis of [disease name]. Findings on an x-ray suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no x-ray findings associated with [disease name]. However, an x-ray may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
Echocardiography or Ultrasound
There are no echocardiography/ultrasound findings associated with [disease name].
OR
Echocardiography/ultrasound may be helpful in the diagnosis of [disease name]. Findings on an echocardiography/ultrasound suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no echocardiography/ultrasound findings associated with [disease name]. However, an echocardiography/ultrasound may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
CT scan
CT scan may be helpful in the diagnosis of Toxoplasmosis. Findings on CT scan suggestive of/diagnostic of [toxoplasmosis] include dilated ventricles with multiple subependymal and parenchymal calcifications (arrow).
OR
There are no CT scan findings associated with [disease name]. However, a CT scan may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
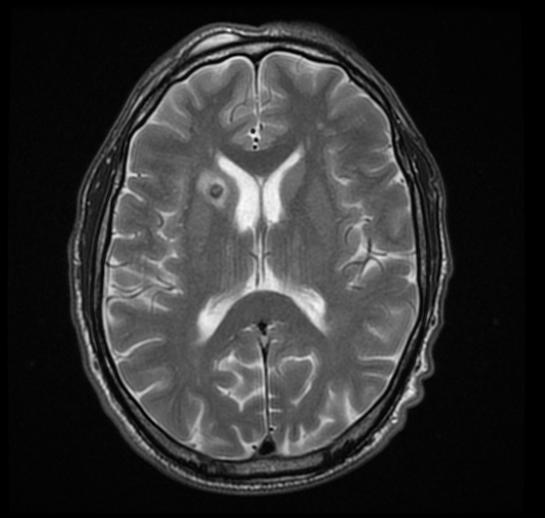
MRI
MRI may be helpful in the diagnosis of [Toxoplasmosis]. Findings on MRI suggestive of/diagnosis include ring enhanced lesion
-
Caption1
Other Imaging Findings
There are no other imaging findings associated with [disease name].
OR
[Imaging modality] may be helpful in the diagnosis of [disease name]. Findings on an [imaging modality] suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
Other Diagnostic Studies
There are no other diagnostic studies associated with [disease name].
OR
[Diagnostic study] may be helpful in the diagnosis of [disease name]. Findings suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
Other diagnostic studies for [disease name] include [diagnostic study 1], which demonstrates [finding 1], [finding 2], and [finding 3], and [diagnostic study 2], which demonstrates [finding 1], [finding 2], and [finding 3].
Treatment
Medical Therapy
- The mainstay of therapy for [ Toxoplasmosis].[8] is
- Mother: immediate administration of spiramycin
- Fetus: When confirmed or highly suspected, switch to pyrimethamine, sulfadiazine, and folinic acid.
- Newborn: pyrimethamine, sulfadiazine, and folinic acid
- The mainstay of therapy for [ Syphilis] is
14 days of IV penicillin G for both pregnant women and newborns
- The mainstay of therapy for [ lysteriosis] is
IV ampicillin and gentamicin (for both mother and newborn)
- The mainstay of therapy for [ Varicella ] is
For pregnant women or newborns with (severe) infection: acyclovir Administer postexposure prophylaxis in newborns if mother displays symptoms of varicella < 5 days before delivery: IgG antibodies (varicella-zoster immune globulin, VZIG)
- The mainstay of therapy for [ Parvovirus B19 ] is
Intrauterine fetal blood transfusion in cases of severe fetal anemia
- The mainstay of therapy for [ Rubella ] is
Intrauterine rubella infection > 16 weeks: reassurance Congenital rubella syndrome: supportive care (based on individual disease manifestations) and surveillance (including monitoring for late-term complication
- The mainstay of therapy for [ congenital CMV ] is
Fetus Severe anemia: intrauterine blood transfusions Thrombocytopenia: platelet transfusions Newborn Supportive therapy of symptoms (e.g., fluid/electrolyte imbalances, anemia, thrombocytopenia, seizures, secondary infections) Ganciclovir, valganciclovir, or foscarnet Mother: valacyclovir is the only therapy approved during pregnancy; trials with CMV specific hyperimmune globulin ongoing.
Surgery
only for herpes simplex [12] :Cesarean section in women with active genital lesions or prodromal symptoms (e.g., burning pain)
Prevention
Minimizing the risk of transmitting a maternal infection to a fetus is often a major concern for parents. The first step is identifying possible maternal infections. Proper prenatal care in many cases allows for early diagnosis and thus early treatment of certain infections, thus improving the newborn's prognosis [13]
A woman's nutritional status may contribute to her ability to fight off infections, particularly in cases of malnutrition . A well-balanced diet rich in nutrients such as folic acid , calcium, iron, zinc, vitamin D, and the B vitamins is recommended for pregnant women. Mothers are recommended to eat approximately 300 additional calories day (above and beyond a normal non pregnancy diet) to support the fetus's growth and development [14]
Prevention f Toxoplasmosis .[8]
- Avoid raw, undercooked, and cured meats.
- Wash hands frequently, especially after touching soil (e.g., during gardening).
- Avoid contact with cat litter.
Prevention of syphilis [15]
- Maternal screening in early pregnancy
- Nationally notifiable condition: Congenital syphilis and syphilitic childbirth must be reported to local or state health department.
Prevention of Listeriosis [16]
- Avoidance of soft cheeses
- Avoidance of potentially contaminated water and food: See “Food and water safety” in food poisoning.
- Nationally notifiable condition: Listeriosis must be reported to the local or state health department.
Prevention of varicella infection [17]
- Immunization of seronegative women before pregnancy
- VZIG in pregnant women without immunity within 10 days of exposure
Prevention of Parvovirus infection (B 19)[18]
- Hand hygiene (frequent hand washing)
- Pregnant women with risk factors for TORCH infection should avoid potentially contaminated workplaces (e.g., schools, pediatric clinics)
Prevention of congenital rubella[19]
- Immunization of seronegative women before pregnancy
- Nationally notifiable condition: Suspected congenital rubella syndrome must be reported to the local or state health department.
Prevention of Toxoplasmosis [20]
- Frequent hand washing, especially after contact with bodily secretions of small children (e.g., diaper changing)
- Avoidance of food sharing with children
- Avoidance of kissing small children on the mouth
Prevention of herpes simplex virus[21]
- Antiviral therapy (acyclovir) beginning at 36 weeks of gestation for individuals with a known history of HSV lesions
- Cesarean section in women with active genital lesions or prodromal symptoms (e.g., burning pain)
prevention of ZIKV include the following:[22]
- Avoidance of travel to ZIKV endemic areas during pregnancy.
- The use of N,N-Diethyl-meta-toluamide, which has been recommended in pregnancy to prevent ZIKV infection,85 long sleeves and pants or permethrin-treated
clothing, and use of mosquito nets and window screens if living in or traveling to an endemic area.
- If living in an endemic area, areas of standing water (such as tires, buckets,planters, etc) should be eliminated because they are a breeding area for
mosquitoes.
- All pregnant women and their partners should receive counseling on prevention measures including avoidance of mosquito bites and sexual transmission.
- If a couple has a male partner and he travels to an area with ZIKV, they should use
condoms or abstain from sexual activity for 6 months (even in the absence of symptoms).
- If a female travels to an area with risk of ZIKV, condoms or abstinence from sexual activity for 8 weeks (even in the absence of symptoms) is recommended.
- If a pregnant patient and her partner travel to or live in an area with ZIKV, condoms should be used each time the couple has sex for the remainder of pregnancy, or they should
abstain from sexual activity.
References
- ↑ [Ostrander, B., & Bale, J. F. (2019). Congenital and perinatal infections. Neonatal Neurology, 133–153. doi:10.1016/b978-0-444-64029-1.00006-0 ], additional text.
- ↑ 2.0 2.1 "Definitions and Indicators in Family Planning. Maternal & Child Health and Reproductive Health" (PDF). Archived from the original (PDF) on 25 January 2012. Unknown parameter
|url-status=ignored (help) By European Regional Office, World Health Organization. Revised March 1999 & January 2001. In turn citing: WHO Geneva, WHA20.19, WHA43.27, Article 23 - ↑ Singh, Meharban (2010). Care of the Newborn. p. 7. Edition 7. ISBN 9788170820536
- ↑ Stewart, Andrew D.; Logsdon, John M.; Kelley, Steven E. (April 2005). "An empirical study of the evolution of virulence under both horizontal and vertical transmission". Evolution. 59 (4): 730–739. doi:10.1554/03-330. ISSN 0014-3820. PMID 15926685. Unknown parameter
|s2cid=ignored (help) - ↑ Joo, Esther; Carmack, Anne; Garcia-Buñuel, Elizabeth; Kelly, Chester J. (February 2000). "Implementation of guidelines for HIV counseling and voluntary HIV testing of pregnant women". American Journal of Public Health. 90 (2): 273–276. doi:10.2105/AJPH.90.2.273. ISSN 0090-0036. PMC 1446152. PMID 10667191.
- ↑ Cavalli-Sforza, Luigi Luca; Feldman, Marcus W. (1981). Cultural Transmission and Evolution: A Quantitative Approach. Monographs in Population Biology. 16. Princeton University Press. pp. 1–388. ISBN 0-691-08283-9. PMID 7300842. Retrieved 30 April 2016.
- ↑ [ https://pediatrics.aappublications.org/content/143/2/e20181487], additional text.
- ↑ 8.0 8.1 8.2 [ Cline, Matthew K., Chasse Bailey-Dorton, and Maria Cayelli. "Update in Maternity Care: Maternal Infections." Clinics in Office Practice 27, no. 1 (March 2000): 13–33. Read more: http://www.healthofchildren.com/P/Perinatal-Infection.html#ixzz6YvWnLQK0]
- ↑ [Ford-Jones, E. Lee, and Greg Ryan. "Implications for the Fetus of Maternal Infections in Pregnancy." In Infectious Diseases , 2nd ed. Edited by Jonathan Cohen et all. New York: Mosby, 2004 ], additional text.
- ↑ [ Lazzarotto T, Guerra B, Gabrielli L, et al. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect 2011;17:1285–93. ], additional text.
- ↑ [ Rabe IB, Staples JE, Villanueva J, et al. Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb Mortal Wkly Rep 2016;65:543–6. ], additional text.
- ↑ [ Riley LE, Wald A. Genital herpes simplex virus infection and pregnancy. In: Post TW, ed. UpToDate. Waltham, MA: UpToDate. http://www.uptodate.com/contents/genital-herpes-simplex-virus-infection-and-pregnancy. Last updated June 18, 2016. Accessed March 22, 2017.], additional text.
- ↑ [Ford-Jones, E. Lee, and Greg Ryan. "Implications for the Fetus of Maternal Infections in Pregnancy." In Infectious Diseases , 2nd ed. Edited by Jonathan Cohen et all. New York: Mosby, 2004. ], additional text.
- ↑ [Ford-Jones, E. Lee, and Greg Ryan. "Implications for the Fetus of Maternal Infections in Pregnancy." In Infectious Diseases , 2nd ed. Edited by Jonathan Cohen et all. New York: Mosby, 2004 ], additional text.
- ↑ [Centers for Disease Control and Prevention. 2017 Nationally Notifiable Conditions. https://wwwn.cdc.gov/nndss/conditions/notifiable/2017/. Updated January 1, 2017. Accessed March 22, 2017. ], additional text.
- ↑ [ Janakiraman V. Listeriosis in pregnancy: diagnosis, treatment, and prevention. Rev Obstet Gynecol. 2008; 1(4): pp. 179–85. pmid: 19173022. ], additional text.
- ↑ [ Centers for Disease Control and Prevention. 2017 Nationally Notifiable Conditions. https://wwwn.cdc.gov/nndss/conditions/notifiable/2017/. Updated January 1, 2017. Accessed March 22, 2017. ], additional text.
- ↑ [ Lamont RF, Sobel JD, Vaisbuch E, et al. Parvovirus B19 infection in human pregnancy. BJOG. 2010; 118(2): pp. 175–186. doi: 10.1111/j.1471-0528.2010.02749.x ], additional text.
- ↑ [ Centers for Disease Control and Prevention. Three Cases of Congenital Rubella Syndrome in the Postelimination Era: Maryland, Alabama, and Illinois, 2012. MMWR Morb Mortal Wkly Rep. 2013; 62(12): pp. 226–229. url: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6212a3.htm. ], additional text.
- ↑ [Demmler-Harrison GJ. Congenital cytomegalovirus infection: Management and outcome. In: Post TW, ed. UpToDate. Waltham, MA: UpToDate. http://www.uptodate.com/contents/congenital-cytomegalovirus-infection-management-and-outcome. Last updated July 29, 2016. Accessed March 22, 2017. ], additional text.
- ↑ [ Demmler-Harrison GJ. Neonatal herpes simplex virus infection: Management and prevention. In: Post TW, ed. UpToDate. Waltham, MA: UpToDate. http://www.uptodate.com/contents/neonatal-herpes-simplex-virus-infection-management-and-prevention. Last updated February 16, 2016. Accessed March 22, 2017. ], additional text.
- ↑ [ Centers for Disease Control and Prevention (CDC). Zika virus prevention. Available at: https://www.cdc.gov/zika/prevention/index.html. Accessed April 24, 2017 ], additional text.