Pathophysiology compartment syndrome
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohammadmain Rezazadehsaatlou[2] ;
Overview
Any condition that results in an increase in compartment contents or reduction in a compartment’s volume could lead to the development of an acute compartment syndrome. When pressure is elevated capillary blood flow is compromised. Edema of the soft tissue within the compartment further raises the intra-compartment pressure, which compromised venous and lymphatic drainage of the injured area. Pressure, if further increased in a reinforcing vicious cycle, can compromise arteriole perfusion, leading to further tissue ischemia.
The normal mean interstitial tissue pressure is 25 mmHg (range 20–30 mmHg), and if it is over 50–60 mmHg or below 10mmHg (or below the diastolic blood pressure minus 20–30 mmHg) functional tissue changes can occur e.g tissue necrosis. . Arteries and arterioles are stable at these pressures, however the tissues within the compartment dependent on the capillaries for nutrients suffer hypoxia.
Untreated compartment syndrome mediated ischemia of the muscles and nerves lead to eventual irreversible damage and death of the tissues within the compartment.
Pathophysiology
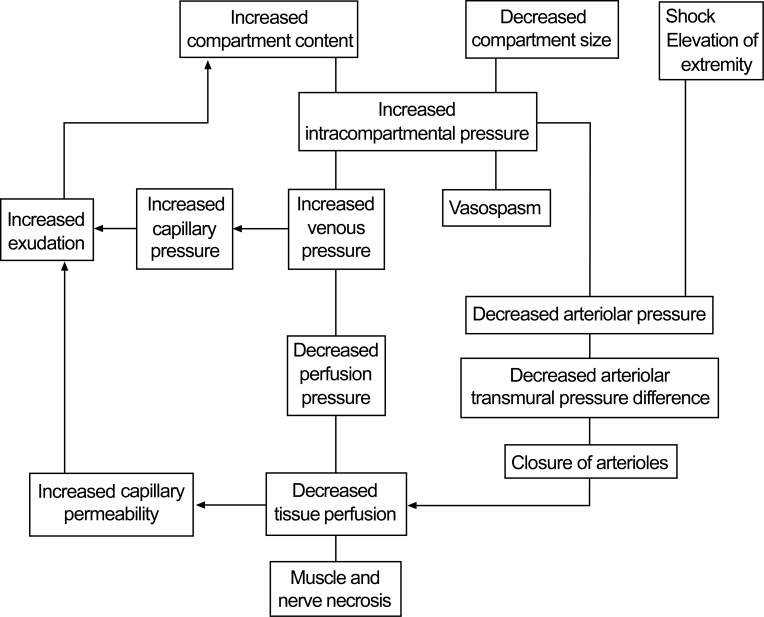
Compartment syndrome is defined as ‘an increased pressure within a confined compartmental space decreasing the perfusion pressure to the tissue. CS is caused by increasing pressure due to the edema and/or hemorrhage within an anatomic compartment bound. Increased pressures leads to the decreased capillary perfusion pressure resulting in sensory disturbance, pain at rest, ischemia, soft tissue compromise, and subsequent necrosis, and even ischemic neuropathy and chronic neuropathic pain. CS is defined as ‘an increased pressure within a confined compartmental space decreasing the perfusion pressure to the tissue. Whenever fluid (from bleeding etc) enters into a fixed volume compartment, both the tissue and venous pressure increase then the capillary collapse with ensuing muscle leading to the nerve ischaemia. A similar reduction in capillary perfusion pressure when the compartment size changes for example during the external compression, then due to an increase in intracompartmental pressure, as well as a reduction in the arteriolar pressure.
Two distinct types of compartment syndrome have been recognized in this regrard:
a: CS associated with trauma as seen in fractures or muscle injuries.
b: CS associated with repetitive loading or microtrauma related to physical activity (also is called exertional compartment syndrome).
Tissue perfusion is proportional to the difference between the capillary perfusion pressure (CPP) and the interstitial fluid pressure, and its evaluated as:
LBF = (PA - PV)/R
(LBF is local blood flow, PA is local arterial pressure, PV is venous pressure, and R is local vascular resistance)
Normal myocyte metabolism requires a 5-7 mm Hg of oxygen tension, which can be provided with a CPP of 25 mm Hg and an interstitial tissue pressure of 4-6 mm Hg.
Whenever in a fixed-volume compartment the fluid occurs then tissue pressure increases and venous pressure rises. When the interstitial pressure exceeds the CPP (a narrowed arteriovenous [AV] perfusion gradient), capillary collapse and muscle and tissue ischemia occur.
Skeletal muscle responds to ischemia by releasing histaminelike substances that increase vascular permeability. Plasma leaks out of the capillaries, and relative blood sludging in the small capillaries occurs, worsening the ischemia. The myocytes begin to lyse, and the myofibrillar proteins decompose into osmotically active particles that attract water from arterial blood.