Parkes Weber syndrome: Difference between revisions
mNo edit summary |
|||
| Line 9: | Line 9: | ||
==Overview== | ==Overview== | ||
'''Parkes Weber Syndrome''' also known as (PKWS) is a rare vascular abnormality characterized by a cutaneous flush with underlying multiple CM (capillary malformation), VM (venuous malformation), LM (lymphatic malformation) and AVFs (arteriovenous fistulas), in association with soft tissue and skeletal hypertrophy of the affected limb. <ref>Eerola, I.; Boon, L. M.; Mulliken, J. B.; Burrows, P. E.; Dompmartin, A.; Watanabe, S.; Vanwijck, R.; Vikkula, M. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am. J. Hum. Genet. 73: 1240-1249, 2003. PMID 14639529</ref> <ref>Mulliken, J. B.; Young, A. E. (eds.): Vascular Birthmarks: Hemangiomas and Vascular Malformations. Philadelphia: W. B. Saunders Co., 1988 </ref><ref name=":0">{{Cite journal|last=Lee BB.|first=|date=2012|title=Klippel-Trenaunay Syndrome: is this term still worthy to use?|url=|journal=Acta Phlebol|volume=13|pages=1-2|via=}}</ref><ref name=":1">{{Cite web|url=https://ghr.nlm.nih.gov/condition/parkes-weber-syndrome#sourcesforpage|title=Genetics home reference|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref> This disease is clinically distinct from Klippel-Trenaunay syndrome but the two are often confused as the presentation is very similar. AVMs present in PWS serve as the hallmark for distinguishing the two syndromes.<ref name=":0" /><ref>{{Cite journal|last=Gloviczki|first=P|date=2009|title=Vascular malformations: an update.|url=https://www.ncbi.nlm.nih.gov/pubmed/19713211|journal=Perspectives in vascular surgery and endovascular therapy|volume=|pages=|via=}}</ref> | '''Parkes Weber Syndrome''' also known as (PKWS) is a rare vascular abnormality characterized by a cutaneous flush with underlying multiple CM (capillary malformation), VM (venuous malformation), LM (lymphatic malformation) and AVFs (arteriovenous fistulas), in association with soft tissue and skeletal hypertrophy of the affected limb. <ref>Eerola, I.; Boon, L. M.; Mulliken, J. B.; Burrows, P. E.; Dompmartin, A.; Watanabe, S.; Vanwijck, R.; Vikkula, M. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am. J. Hum. Genet. 73: 1240-1249, 2003. PMID 14639529</ref> <ref>Mulliken, J. B.; Young, A. E. (eds.): Vascular Birthmarks: Hemangiomas and Vascular Malformations. Philadelphia: W. B. Saunders Co., 1988 </ref><ref name=":0">{{Cite journal|last=Lee BB.|first=|date=2012|title=Klippel-Trenaunay Syndrome: is this term still worthy to use?|url=|journal=Acta Phlebol|volume=13|pages=1-2|via=}}</ref><ref name=":1">{{Cite web|url=https://ghr.nlm.nih.gov/condition/parkes-weber-syndrome#sourcesforpage|title=Genetics home reference|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref> Discovered by Frederick P. Weber in 1907, who noted two patients with port-wine stains and enlargement of the limb with accompanying enlargement of the vasculature leading to a palpable thrill. Malformations can be present since birth, and in some cases are controlled by genetic mutations transferred in an autosomal dominant pattern. This disease is clinically distinct from Klippel-Trenaunay syndrome but the two are often confused as the presentation is very similar. AVMs present in PWS serve as the hallmark for distinguishing the two syndromes.<ref name=":0" /><ref>{{Cite journal|last=Gloviczki|first=P|date=2009|title=Vascular malformations: an update.|url=https://www.ncbi.nlm.nih.gov/pubmed/19713211|journal=Perspectives in vascular surgery and endovascular therapy|volume=|pages=|via=}}</ref> | ||
Recently a disorder in the RASA1 gene has been implicated in development of this syndrome.<ref>{{Cite journal|last=N|first=Revencu|date=2008|title=Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations.|url=https://www.ncbi.nlm.nih.gov/pubmed/18446851|journal=Hum mutat|volume=29|pages=959-965|via=}}</ref> F. Parkes Weber's (1863-1962) name is also attached to hereditary hemorrhagic telangiectasia, Sturge-Weber syndrome, Weber-Christian disease, and Klippel-Trenaunay-Weber syndrome.<br /> | |||
==Historical Perspective== | ==Historical Perspective== | ||
*Parkes-Weber Syndrome was first discovered by Frederick Parkes Weber, an English dermatologist, in 1907. | *Parkes-Weber Syndrome was first discovered by Frederick Parkes Weber, an English dermatologist, in 1907. | ||
*In | *In 2008, RASA1 mutations were first comfirmed in the pathogenesis of this syndrome, along with other fast-flow vascular syndromes. | ||
* | *There is no known cure for PWS, although symptomatic control is available in the form of embolization and corrective surgery depending on the implicit malformations. | ||
==Pathophysiology== | ==Pathophysiology== | ||
Gene map locus is 5q13. | Gene map locus is 5q13.1<ref name=":2">{{Cite journal|last=SZELIGA|first=Adrianna|date=September 2019|title=Parkes Weber Syndrome.|url=http://www.ojs.ukw.edu.pl/index.php/johs/article/view/7441|journal=Journal of Health, education and sport|volume=|pages=|via=}}</ref>. | ||
Six families reported by Eerola et al. in 2003, manifested atypical capillary malformations associated with either arteriovenous malformation, arteriovenous fistula, or Parkes Weber syndrome. They named this association CM-AVM for 'capillary malformation-arteriovenous malformation' and found mutation in the RASA1 gene in affected members of these families. | Six families reported by Eerola et al. in 2003, manifested atypical capillary malformations associated with either arteriovenous malformation, arteriovenous fistula, or Parkes Weber syndrome. They named this association CM-AVM for 'capillary malformation-arteriovenous malformation' and found mutation in the RASA1 gene in affected members of these families. | ||
*The pathogenesis | *The pathogenesis is not well understood at this time, but theories have been put forward in literature: | ||
* | **Congenital blockage of deep veins draining the affected limb | ||
**Germ layer (mesodermal) irregularity leading to abnormal development of soft tissue and subsequent development of high flow AVMs. | |||
*On | **RASA1 gene mutation has been associated with the development of PWS, involving the tyrosine kinase pathway. This gene is responsible for regulation of cellular growth and differentiation. It codes for the p120-RasGAP protein responsible for various growth factor receptors controlling stem cell migration and proliferation. In this disease the mutated gene codes for a non-functioning protein. | ||
*On gross pathology, capillary malformations, high flow arteriovenous fistulas, VMs and LMs are characteristic. | |||
==Clinical Features== | ==Clinical Features== | ||
PWS can present with: | PWS can present with: | ||
* Upper or lower limb hypertrophy | * Upper or lower limb hypertrophy. More commonly the lower limb is involved. Patients with AVFs (arteriovenuous fistula) have a greater degree of hypertrophy. | ||
*Bruit or thrill over the affected limb. | |||
* Abnormal bleeding/ recurrent bleeding from skin lesions | * Abnormal bleeding/ recurrent bleeding from skin lesions | ||
* Large, flat, pink discolouration on the skin known as a port-wine stain (naevus flammeus) | * Large, flat, pink discolouration on the skin known as a port-wine stain (naevus flammeus) | ||
* Enlarged | * Enlarged veins showing evidence of high flow AV shunting. | ||
* Capillary malformations resulting in petecheia on face, arms and legs | * Capillary malformations resulting in petecheia on face, arms and legs | ||
*[[Telangiectasia]] | *[[Telangiectasia]] | ||
*Venous malformations | *Venous malformations | ||
*Varicose veins | *Varicose veins | ||
| Line 56: | Line 47: | ||
*Parkes Weber Syndrome must be differentiated from other diseases that cause port-wine stains and capillary malformations, such as: | *Parkes Weber Syndrome must be differentiated from other diseases that cause port-wine stains and capillary malformations, such as: | ||
:*Klippel-Trenaunay Syndrome | :*Klippel-Trenaunay Syndrome: KTS consists of the following triad of capillary malformation, venous malformation and lymphatic malformation. AVMs are present only in PWS. | ||
:*'''[[Sturge-Weber syndrome]]:''' Sturge-Weber syndrome is characterized by a triad of facial port wine | :*'''[[Sturge-Weber syndrome]]:''' Sturge-Weber syndrome is characterized by a triad of facial port wine stains (classically the area of the ophthalmic and/or maxillary branch (segments V1/V2) of the trigeminal nerve), leptomeningeal angiomatosis, and ocular involvement. The capillary malformation associated with SWS usually presents as both upper and lower eyelid staining, and is often bilateral.<ref>{{Cite journal|last=Tallman|first=B|date=1991|title=Location of port-wine stains and the likelihood of ophthalmic and/or central nervous system complications.|url=|journal=Pediatrics|volume=|pages=|via=}}</ref> | ||
:*'''[[Proteus syndrome]]''' — Proteus syndrome is an extremely rare disorder characterized by random overgrowth of body parts. The cause is considered to be mosaicism for a somatic activating mutation in the ''AKT1'' oncogene. | :*'''[[Proteus syndrome]]''' — Proteus syndrome is an extremely rare disorder characterized by random overgrowth of body parts. The cause is considered to be mosaicism for a somatic activating mutation in the ''AKT1'' oncogene. | ||
:*'''[[CLOVES syndrome|CLOVES]] syndrome''' | :*'''[[CLOVES syndrome|CLOVES]] syndrome''' | ||
| Line 68: | Line 59: | ||
*Early clinical features include | *Early clinical features include | ||
*If left untreated, | ** a capillary malformation resulting in pink, warm birthmarks flush with the skin called a port-wine stain | ||
** frequent or recurrent bleeding from malformations near the skin surface | |||
*Prognosis is generally | ** cellulitis | ||
** varying degrees of hypertrophy of a limb (usually a leg) | |||
** pain in the affected limb which varies in severity | |||
** increased blood flow through an arteriovenous malformation leading to cardiac issues | |||
*If left untreated, patients with PWS progress to fatal high output heart failure, or excessive bleeding can be cause of death. Other complications include cutaneous ischemia, cardiac hypertrophy and limb amputation.<ref name=":2" /> | |||
*Prognosis is generally fair. As PWS is a progressive condition, management depends on the extent of the overgrowth and the problems it causes. Any comorbidities, the condition of the patient's heart, refractory nature of the disease, overall health of the patient influence outcomes along with timely diagnosis and intervention. Overgrowth is progressive until epiphyseal closure. | |||
== Diagnosis == | == Diagnosis == | ||
*Diagnosis of PWS is a difficult process and is frequently misdiagnosed as KTS. It is made on clinical grounds. A combination of cutaneous capillary-Lymphatic-venuous malformations along with arterio-venous malformations as the main defect define the syndrome. | |||
* | |||
===Imaging Findings=== | ===Imaging Findings=== | ||
* | *Imaging modalities useful to diagnose and map vascular malformations include: | ||
*** '''Magnetic resonance imaging (MRI):''' This is a high-resolution scan employed to gauge the extent of the hypertrophy (overgrowth) of tissue and provides insight to the problems due to it. Subsequently, MR projection angiography may be used to distinguish PWS from KTS noninvasively. | |||
* | *** '''Ultrasound (also called ultrasonography):''' An ultrasound helps to visualise vascular system abnormalities and can determine the blood flow through the AVM. Antenatal testing can sometimes allude to malformations. | ||
* | *** '''Computerized tomography scan (also called a CT or CAT scan):''' A CT scan shows detailed images of the area in slices and is especially helpful for evaluating the bones in the affected limb. | ||
* | *** '''Angiogram''': A dye is injected which outlines the vasculature, providing a detailed view of the blood vessels in the affected limb. This is the gold standard to visualise the location and degree of AVMs. Digital Subtraction Angiography can be used to differentiate between high and low flow AVMs. This technique is used mostly for patients who are candidates for embolization therapy. | ||
*** '''Echocardiogram:''' To check the condition of the patients heart. | |||
* | |||
* | |||
<br /> | |||
== Treatment == | == Treatment == | ||
=== Medical Therapy === | === Medical Therapy === | ||
* There is no treatment for PWS; the mainstay of therapy is supportive care. | * There is no treatment for PWS; the mainstay of therapy is supportive care. Depending on the extent of malformations, embolization can be done to block off AVMs and to prevent overgrowth of limbs. | ||
*The use of compressive stockings can prevent edema and minimize discomfort.<ref name=":2" /> | |||
=== Surgery === | === Surgery === | ||
* | *Embolization in conjunction with removal of AVMs is the most common approach to the treatment of PWS.<ref>{{Cite journal|last=Banzic|first=I|date=2017 July|title=Parkes Weber syndrome—Diagnostic and management paradigms: A systematic review|url=https://www.ncbi.nlm.nih.gov/pubmed/27511883|journal=Phlebology|volume=|pages=|via=}}</ref> | ||
* | *A recent case report suggests early amputation of the limb as having better outcomes than placing stents in high flow AVMs.<ref>{{Cite journal|last=Acar|first=Zeydin|date=|title=Short- and mid-term effects of covered stent implantation on extremity findings and heart failure in Parkes Weber syndrome: a case report|url=https://academic.oup.com/ehjcr/advance-article/doi/10.1093/ehjcr/ytaa046/5802614|journal=European Heart Journal - Case Reports|volume=|pages=|via=}}</ref> | ||
=== Prevention === | === Prevention === | ||
*PWS is a genetic disease as such there are no primary preventive measures available. | *PWS is a genetic disease as such there are no primary preventive measures available. | ||
<br /> | |||
===Multi Sliced CT=== | ===Multi Sliced CT=== | ||
<div align="center"> | <div align="center"> | ||
Latest revision as of 17:14, 5 April 2020
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
Parkes Weber Syndrome also known as (PKWS) is a rare vascular abnormality characterized by a cutaneous flush with underlying multiple CM (capillary malformation), VM (venuous malformation), LM (lymphatic malformation) and AVFs (arteriovenous fistulas), in association with soft tissue and skeletal hypertrophy of the affected limb. [1] [2][3][4] Discovered by Frederick P. Weber in 1907, who noted two patients with port-wine stains and enlargement of the limb with accompanying enlargement of the vasculature leading to a palpable thrill. Malformations can be present since birth, and in some cases are controlled by genetic mutations transferred in an autosomal dominant pattern. This disease is clinically distinct from Klippel-Trenaunay syndrome but the two are often confused as the presentation is very similar. AVMs present in PWS serve as the hallmark for distinguishing the two syndromes.[3][5]
Recently a disorder in the RASA1 gene has been implicated in development of this syndrome.[6] F. Parkes Weber's (1863-1962) name is also attached to hereditary hemorrhagic telangiectasia, Sturge-Weber syndrome, Weber-Christian disease, and Klippel-Trenaunay-Weber syndrome.
Historical Perspective
- Parkes-Weber Syndrome was first discovered by Frederick Parkes Weber, an English dermatologist, in 1907.
- In 2008, RASA1 mutations were first comfirmed in the pathogenesis of this syndrome, along with other fast-flow vascular syndromes.
- There is no known cure for PWS, although symptomatic control is available in the form of embolization and corrective surgery depending on the implicit malformations.
Pathophysiology
Gene map locus is 5q13.1[7].
Six families reported by Eerola et al. in 2003, manifested atypical capillary malformations associated with either arteriovenous malformation, arteriovenous fistula, or Parkes Weber syndrome. They named this association CM-AVM for 'capillary malformation-arteriovenous malformation' and found mutation in the RASA1 gene in affected members of these families.
- The pathogenesis is not well understood at this time, but theories have been put forward in literature:
- Congenital blockage of deep veins draining the affected limb
- Germ layer (mesodermal) irregularity leading to abnormal development of soft tissue and subsequent development of high flow AVMs.
- RASA1 gene mutation has been associated with the development of PWS, involving the tyrosine kinase pathway. This gene is responsible for regulation of cellular growth and differentiation. It codes for the p120-RasGAP protein responsible for various growth factor receptors controlling stem cell migration and proliferation. In this disease the mutated gene codes for a non-functioning protein.
- On gross pathology, capillary malformations, high flow arteriovenous fistulas, VMs and LMs are characteristic.
Clinical Features
PWS can present with:
- Upper or lower limb hypertrophy. More commonly the lower limb is involved. Patients with AVFs (arteriovenuous fistula) have a greater degree of hypertrophy.
- Bruit or thrill over the affected limb.
- Abnormal bleeding/ recurrent bleeding from skin lesions
- Large, flat, pink discolouration on the skin known as a port-wine stain (naevus flammeus)
- Enlarged veins showing evidence of high flow AV shunting.
- Capillary malformations resulting in petecheia on face, arms and legs
- Telangiectasia
- Venous malformations
- Varicose veins
- Congestive heart failure
Differentiating Parkes-Weber Syndrome from other Diseases
- Parkes Weber Syndrome must be differentiated from other diseases that cause port-wine stains and capillary malformations, such as:
- Klippel-Trenaunay Syndrome: KTS consists of the following triad of capillary malformation, venous malformation and lymphatic malformation. AVMs are present only in PWS.
- Sturge-Weber syndrome: Sturge-Weber syndrome is characterized by a triad of facial port wine stains (classically the area of the ophthalmic and/or maxillary branch (segments V1/V2) of the trigeminal nerve), leptomeningeal angiomatosis, and ocular involvement. The capillary malformation associated with SWS usually presents as both upper and lower eyelid staining, and is often bilateral.[8]
- Proteus syndrome — Proteus syndrome is an extremely rare disorder characterized by random overgrowth of body parts. The cause is considered to be mosaicism for a somatic activating mutation in the AKT1 oncogene.
- CLOVES syndrome
- Capillary malformation-arteriovenous malformation syndrome
Epidemiology and Demographics
- Exact prevalence of PWS is unknown.[4]
Natural History, Complications and Prognosis
- Early clinical features include
- a capillary malformation resulting in pink, warm birthmarks flush with the skin called a port-wine stain
- frequent or recurrent bleeding from malformations near the skin surface
- cellulitis
- varying degrees of hypertrophy of a limb (usually a leg)
- pain in the affected limb which varies in severity
- increased blood flow through an arteriovenous malformation leading to cardiac issues
- If left untreated, patients with PWS progress to fatal high output heart failure, or excessive bleeding can be cause of death. Other complications include cutaneous ischemia, cardiac hypertrophy and limb amputation.[7]
- Prognosis is generally fair. As PWS is a progressive condition, management depends on the extent of the overgrowth and the problems it causes. Any comorbidities, the condition of the patient's heart, refractory nature of the disease, overall health of the patient influence outcomes along with timely diagnosis and intervention. Overgrowth is progressive until epiphyseal closure.
Diagnosis
- Diagnosis of PWS is a difficult process and is frequently misdiagnosed as KTS. It is made on clinical grounds. A combination of cutaneous capillary-Lymphatic-venuous malformations along with arterio-venous malformations as the main defect define the syndrome.
Imaging Findings
- Imaging modalities useful to diagnose and map vascular malformations include:
- Magnetic resonance imaging (MRI): This is a high-resolution scan employed to gauge the extent of the hypertrophy (overgrowth) of tissue and provides insight to the problems due to it. Subsequently, MR projection angiography may be used to distinguish PWS from KTS noninvasively.
- Ultrasound (also called ultrasonography): An ultrasound helps to visualise vascular system abnormalities and can determine the blood flow through the AVM. Antenatal testing can sometimes allude to malformations.
- Computerized tomography scan (also called a CT or CAT scan): A CT scan shows detailed images of the area in slices and is especially helpful for evaluating the bones in the affected limb.
- Angiogram: A dye is injected which outlines the vasculature, providing a detailed view of the blood vessels in the affected limb. This is the gold standard to visualise the location and degree of AVMs. Digital Subtraction Angiography can be used to differentiate between high and low flow AVMs. This technique is used mostly for patients who are candidates for embolization therapy.
- Echocardiogram: To check the condition of the patients heart.
Treatment
Medical Therapy
- There is no treatment for PWS; the mainstay of therapy is supportive care. Depending on the extent of malformations, embolization can be done to block off AVMs and to prevent overgrowth of limbs.
- The use of compressive stockings can prevent edema and minimize discomfort.[7]
Surgery
- Embolization in conjunction with removal of AVMs is the most common approach to the treatment of PWS.[9]
- A recent case report suggests early amputation of the limb as having better outcomes than placing stents in high flow AVMs.[10]
Prevention
- PWS is a genetic disease as such there are no primary preventive measures available.
Multi Sliced CT
-
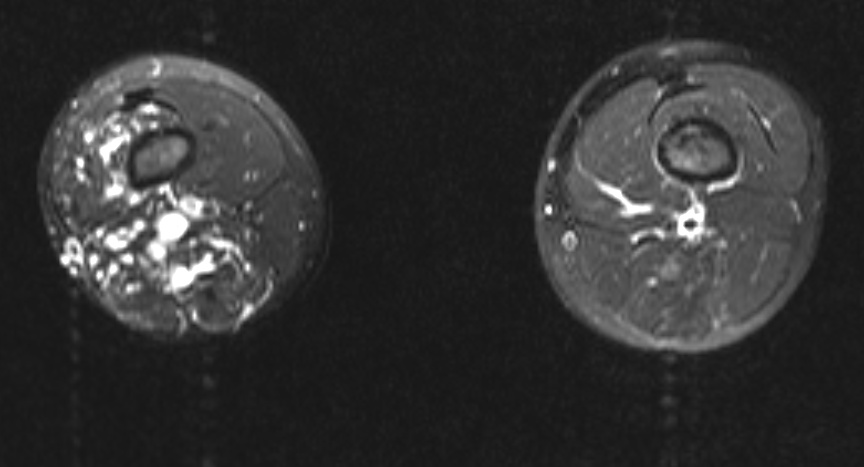
Multi Sliced CT - Lower extremities: A 10 year old boy presented for assessment of leg length discrepancy and cutaneous “capillary” vascular malformation. The provisional diagnosis was Klippel-Trenaunay syndrome. The axial fat-saturated T2 weighted MRI above shows dilated vascular structures in the right lower limb involving subcutaneous and multiple muscle compartments. Flow voids and pulsation artefact (particularly laterally) suggest a high flow component consistent with arteriovenous malformation. These findings favour Parkes Weber syndrome over Klippel-Trenaunay syndrome. There was no high output cardiac failure in this case. (Image courtesy of Dr Laughlin Dawes)
See Also
References
- ↑ Eerola, I.; Boon, L. M.; Mulliken, J. B.; Burrows, P. E.; Dompmartin, A.; Watanabe, S.; Vanwijck, R.; Vikkula, M. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am. J. Hum. Genet. 73: 1240-1249, 2003. PMID 14639529
- ↑ Mulliken, J. B.; Young, A. E. (eds.): Vascular Birthmarks: Hemangiomas and Vascular Malformations. Philadelphia: W. B. Saunders Co., 1988
- ↑ 3.0 3.1 Lee BB. (2012). "Klippel-Trenaunay Syndrome: is this term still worthy to use?". Acta Phlebol. 13: 1–2.
- ↑ 4.0 4.1 "Genetics home reference".
- ↑ Gloviczki, P (2009). "Vascular malformations: an update". Perspectives in vascular surgery and endovascular therapy.
- ↑ N, Revencu (2008). "Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations". Hum mutat. 29: 959–965.
- ↑ 7.0 7.1 7.2 SZELIGA, Adrianna (September 2019). "Parkes Weber Syndrome". Journal of Health, education and sport.
- ↑ Tallman, B (1991). "Location of port-wine stains and the likelihood of ophthalmic and/or central nervous system complications". Pediatrics.
- ↑ Banzic, I (2017 July). "Parkes Weber syndrome—Diagnostic and management paradigms: A systematic review". Phlebology. Check date values in:
|date=(help) - ↑ Acar, Zeydin. "Short- and mid-term effects of covered stent implantation on extremity findings and heart failure in Parkes Weber syndrome: a case report". European Heart Journal - Case Reports.