Palivizumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Palivizumab is a monoclonal antibody that is FDA approved for the {{{indicationType}}} of prevention of serious lower respiratory tract disease caused by RSV in children at high risk of RSV disease. Common adverse reactions include fever and rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Palivizumab in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Palivizumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Synagis is indicated for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in children at high risk of RSV disease.
- The following points should be considered when prescribing Synagis:
- Safety and efficacy were established in children with bronchopulmonary dysplasia (BPD), infants with a history of premature birth (less than or equal to 35 weeks gestational age), and children with hemodynamically significant congenital heart disease (CHD) [see Clinical Studies (14)].
- The safety and efficacy of Synagis have not been established for treatment of RSV disease.
- The recommended dose of Synagis is 15 mg per kg of body weight given monthly by intramuscular injection. The first dose of Synagis should be administered prior to commencement of the RSV season and the remaining doses should be administered monthly throughout the RSV season. Children who develop an RSV infection should continue to receive monthly doses throughout the RSV season. In the northern hemisphere, the RSV season typically commences in November and lasts through April, but it may begin earlier or persist later in certain communities.
- Synagis serum levels are decreased after cardio-pulmonary bypass [see Clinical Pharmacology (12.3)]. Children undergoing cardio-pulmonary bypass should receive an additional dose of Synagis as soon as possible after the cardio-pulmonary bypass procedure (even if sooner than a month from the previous dose). Thereafter, doses should be administered monthly as scheduled.
- The efficacy of Synagis at doses less than 15 mg per kg, or of dosing less frequently than monthly throughout the RSV season, has not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Palivizumab in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Palivizumab in pediatric patients.
Contraindications
- Synagis is contraindicated in children who have had a previous significant hypersensitivity reaction to Synagis.
Warnings
Precautions
- Hypersensitivity Reactions
- Cases of anaphylaxis and anaphylactic shock, including fatal cases, have been reported following initial exposure or re-exposure to Synagis. Other acute hypersensitivity reactions, which may be severe, have also been reported on initial exposure or re-exposure to Synagis. Signs and symptoms may include urticaria, pruritus, angioedema, dyspnea, respiratory failure, cyanosis, hypotonia, hypotension, and unresponsiveness. The relationship between these reactions and the development of antibodies to Synagis is unknown. If a significant hypersensitivity reaction occurs with Synagis, its use should be permanently discontinued. If anaphylaxis or other significant hypersensitivity reaction occurs, administer appropriate medications (e.g., epinephrine) and provide supportive care as required. If a mild hypersensitivity reaction occurs, clinical judgment should be used regarding cautious readministration of Synagis.
- Coagulation Disorders
- Synagis is for intramuscular use only. As with any intramuscular injection, Synagis should be given with caution to children with thrombocytopenia or any coagulation disorder.
- RSV Diagnostic Test Interference
- Palivizumab may interfere with immunological-based RSV diagnostic tests such as some antigen detection-based assays. In addition, palivizumab inhibits virus replication in cell culture, and therefore may also interfere with viral culture assays. Palivizumab does not interfere with reverse transcriptase-polymerase chain reaction based assays. Assay interference could lead to false-negative RSV diagnostic test results. Therefore, diagnostic test results, when obtained, should be used in conjunction with clinical findings to guide medical decisions [see Microbiology (12.4)].
- Treatment of RSV Disease
- The safety and efficacy of Synagis have not been established for treatment of RSV disease.
- Proper Administration
- The single-dose vial of Synagis does not contain a preservative. Administration of Synagis should occur immediately after dose withdrawal from the vial. The vial should not be re-entered. Discard any unused portion.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
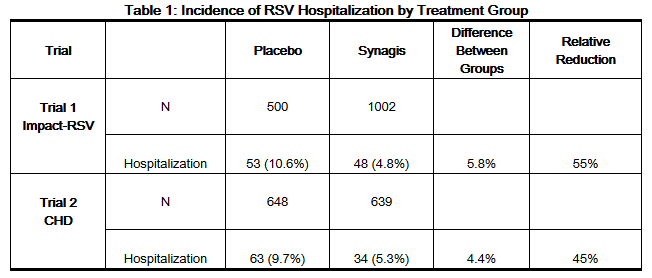
- The data described below reflect exposure to Synagis (n=1639) compared with placebo (n=1143) in children 3 days to 24.1 months of age at high risk of RSV-related hospitalization in two clinical trials. Trial 1 was conducted during a single RSV season and studied a total of 1502 children less than or equal to 24 months of age with BPD or infants with premature birth (less than or equal to 35 weeks gestation) who were less than or equal to 6 months of age at study entry. Trial 2 was conducted over four consecutive seasons among a total of 1287 children less than or equal to 24 months of age with hemodynamically significant congenital heart disease.
- In Trials 1 and 2 combined, fever and rash were each reported more frequently among Synagis than placebo recipients, 27% versus 25%, and 12% versus 10%, respectively. Adverse reactions observed in the 153-patient crossover study comparing the liquid and lyophilized formulations were comparable for the two formulations, and were similar to those observed with Synagis in Trials 1 and 2.
- Immunogenicity
- In Trial 1, the incidence of anti-palivizumab antibody following the fourth injection was 1.1% in the placebo group and 0.7% in the Synagis group. In children receiving Synagis for a second season, one of the fifty-six children had transient, low titer reactivity. This reactivity was not associated with adverse events or alteration in serum concentrations. Immunogenicity was not assessed in Trial 2.
- A trial of high-risk preterm children less than or equal to 24 months of age was conducted to evaluate the immunogenicity of the lyophilized formulation of Synagis (used in Trials 1 and 2 above) and the liquid formulation of Synagis. Three hundred seventy-nine children contributed to the 4 to 6 months post-final dose analysis. The rate of anti-palivizumab antibodies at this time point was low in both formulation groups (anti-palivizumab antibodies were not detected in any subject in the liquid formulation group and were detected in one subject in the lyophilized group (0.5%), with an overall rate of 0.3% for both treatment groups combined).
- These data reflect the percentage of children whose test results were considered positive for antibodies to palivizumab in an enzyme-linked immunosorbent assay (ELISA) and are highly dependent on the sensitivity and specificity of the assay.
- The ELISA has substantial limitations in detecting anti-palivizumab antibodies in the presence of palivizumab. Immunogenicity samples tested with the ELISA assay likely contained palivizumab at levels that may interfere with the detection of anti-palivizumab antibodies.
- An electrochemical luminescence (ECL) based immunogenicity assay, with a higher tolerance for palivizumab presence compared to the ELISA, was used to evaluate the presence of anti-palivizumab antibodies in subject samples from two additional clinical trials. The rates of anti-palivizumab antibody positive results in these trials were 1.1% and 1.5%.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of Synagis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: severe thrombocytopenia (platelet count less than 50,000 per microliter)
- General Disorders and Administration Site Conditions: injection site reactions
- Limited information from post-marketing reports suggests that, within a single RSV season, adverse events after a sixth or greater dose of Synagis are similar in character and frequency to those after the initial five doses.
Drug Interactions
- No formal drug-drug interaction studies were conducted. In Trial 1, the proportions of children in the placebo and Synagis groups who received routine childhood vaccines, influenza vaccine, bronchodilators, or corticosteroids were similar and no incremental increase in adverse reactions was observed among children receiving these agents.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Synagis is not indicated for adult usage. It is not known whether Synagis can cause fetal harm or could affect reproductive capacity when administered to a pregnant woman.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Palivizumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Palivizumab during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Palivizumab with respect to nursing mothers.
Pediatric Use
- The safety and effectiveness of Synagis in children greater than 24 months of age at the start of dosing have not been established.
Geriatic Use
There is no FDA guidance on the use of Palivizumab with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Palivizumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Palivizumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Palivizumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Palivizumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Palivizumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Palivizumab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Palivizumab in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Palivizumab in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Palivizumab in the drug label.
Pharmacology
There is limited information regarding Palivizumab Pharmacology in the drug label.
Mechanism of Action
Structure
- Palivizumab is a humanized monoclonal antibody (IgG1к) produced by recombinant DNA technology, directed to an epitope in the A antigenic site of the F protein of RSV. Palivizumab is a composite of human (95%) and murine (5%) antibody sequences. The human heavy chain sequence was derived from the constant domains of human IgG1 and the variable framework regions of the VH genes Cor and Cess. The human light chain sequence was derived from the constant domain of Cк and the variable framework regions of the VL gene K104 with Jк -4. The murine sequences were derived from a murine monoclonal antibody, Mab 1129, in a process that involved the grafting of the murine complementarity determining regions into the human antibody frameworks. Palivizumab is composed of two heavy chains and two light chains and has a molecular weight of approximately 148,000 Daltons.
- Synagis is supplied as a sterile, preservative-free liquid solution at 100 mg per mL to be administered by intramuscular injection. Thimerosal or other mercury-containing salts are not used in the production of Synagis. The solution has a pH of 6.0 and should appear clear or slightly opalescent.
- Each 100 mg single-dose vial of Synagis liquid solution contains 100 mg of palivizumab and also contains chloride (0.5 mg), glycine (0.1 mg), and histidine (3.9 mg), in a volume of 1 mL.
- Each 50 mg single-dose vial of Synagis liquid solution contains 50 mg of palivizumab and also contains chloride (0.2 mg), glycine (0.06 mg), and histidine (1.9 mg), in a volume of 0.5 mL.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Palivizumab in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Palivizumab in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Palivizumab in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Palivizumab in the drug label.
How Supplied
Storage
There is limited information regarding Palivizumab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Palivizumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Palivizumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Palivizumab in the drug label.
Precautions with Alcohol
- Alcohol-Palivizumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Palivizumab |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Palivizumab |Label Name=Palivizumab11.png
}}
{{#subobject:
|Label Page=Palivizumab |Label Name=Palivizumab11.png
}}