Osteosarcoma: Difference between revisions
| Line 41: | Line 41: | ||
==Treatment== | ==Treatment== | ||
[[Osteosarcoma medical therapy|Medical therapy]] | [[Osteosarcoma surgery|Surgical options]] | [[Osteosarcoma primary prevention|Primary prevention]] | [[Osteosarcoma secondary prevention|Secondary prevention]] | [[Osteosarcoma cost-effectiveness of therapy|Financial costs]] | [[Osteosarcoma future or investigational therapies|Future therapies]] | [[Osteosarcoma medical therapy|Medical therapy]] | [[Osteosarcoma surgery|Surgical options]] | [[Osteosarcoma primary prevention|Primary prevention]] | [[Osteosarcoma secondary prevention|Secondary prevention]] | [[Osteosarcoma cost-effectiveness of therapy|Financial costs]] | [[Osteosarcoma future or investigational therapies|Future therapies]] | ||
==Prognosis== | ==Prognosis== | ||
Revision as of 14:57, 18 January 2012
| Osteosarcoma | |
| ICD-10 | C40-C41 |
|---|---|
| ICD-9 | 170 |
| ICD-O: | Template:ICDO |
| OMIM | 259500 |
| DiseasesDB | 9392 |
| MedlinePlus | 001650 |
| MeSH | D012516 |
|
Osteosarcoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Osteosarcoma On the Web |
|
American Roentgen Ray Society Images of Osteosarcoma |
For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Historical Perspective
Pathophysiology
Epidemiology & Demographics
Risk Factors
Screening
Causes
Differentiating Osteosarcoma
Complications & Prognosis
Diagnosis
History and Symptoms | Physical Examination | Staging | Laboratory tests | Electrocardiogram | X Rays | CT | MRI Echocardiography or Ultrasound | Other images | Alternative diagnostics
Treatment
Medical therapy | Surgical options | Primary prevention | Secondary prevention | Financial costs | Future therapies
Prognosis
- Prognosis is separated into three groups.
- Stage I osteosarcoma is rare and includes parosteal osteosarcoma or low-grade central osteosarcoma. It has an excellent prognosis (>90%) with wide resection.
- Stage IIb prognosis depends on the site of the tumor (proximal tibia, femur, pelvis, etc.) size of the tumor mass (in cm.), the degree of necrosis from neoadjuvant chemotherapy (beforeoperation chemotherapy), and pathological factors like the degree of p-glycoprotein, whether your tumor is cxcr4-positive.[1] Her2-positive as these can lead to distant metastases to the lung. Longer time to metastases, more than 12 months or 24 months and the number of metastases and resectability of them lead to the best prognosis with metastatic osteosarcoma. It is better to have fewer metastases than longer time to metastases. Those with a longer length of time(>24months) and few nodules (2 or fewer) have the best prognosis with a 2-year survival after the metastases of 50% 5-year of 40% and 10 year 20%. If metastases are both local and regional the prognosis is different unfortunately.
- Initial Presentation of stage III osteosarcoma with lung metastates depends on the resectability of the primary tumor and lung nodules, degree of necrosis of the primary tumor, and maybe the number of metastases. Overall prognosis is 30% or greater depending.
Case Examples
Case #1
Clinical Summary
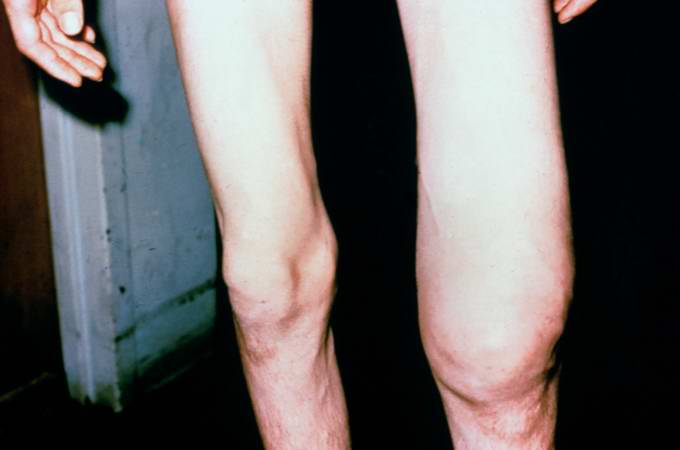
This 14-year-old white male first experienced mild pain in the left knee after playing baseball, approximately two months prior to admission. The pain persisted in an intermittent fashion, and was described as being somewhat worse at night. Approximately two weeks prior to admission, the pain increased significantly and was accompanied by marked swelling and loss of considerable motion of the knee joint. These symptoms were accompanied by a history of decreased appetite, lethargy, and a 10-pound weight loss. On physical examination, the left knee was enlarged diffusely, firm, and non-tender. Following biopsy, the patient was subjected to surgical removal of the distal femur and knee with placement of a prosthetic knee joint and bone grafts.
Autopsy Findings
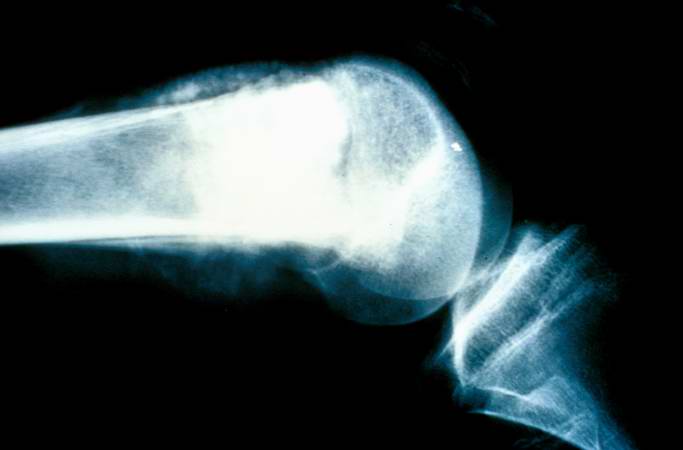
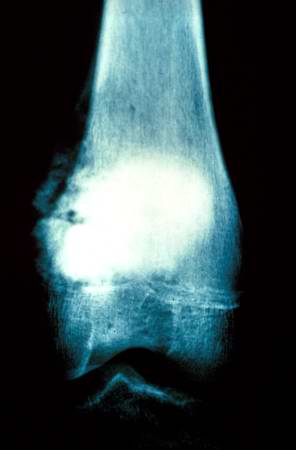
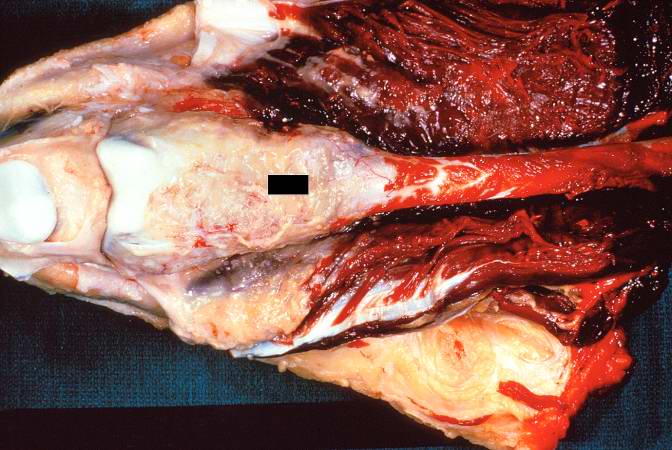
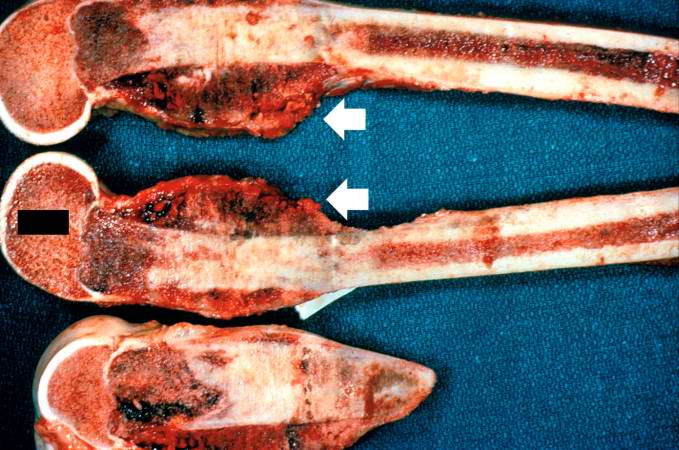
The distal diaphysis of the femur and adjacent soft tissues were involved in a 15 x 10 x 10-cm mass. The cut surface of the mass was fleshy white, with focal areas of hemorrhage.
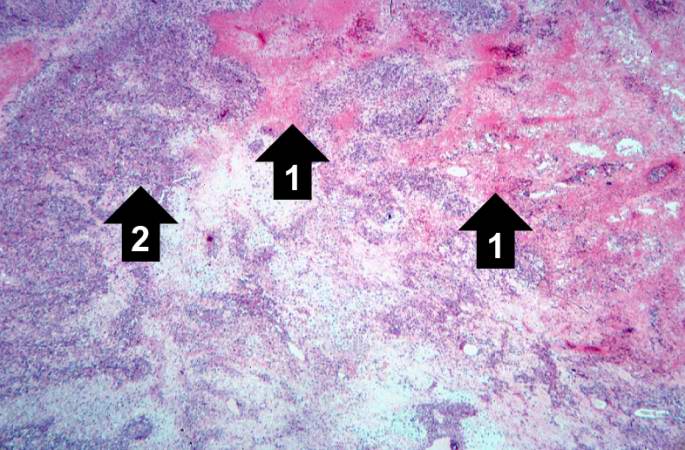
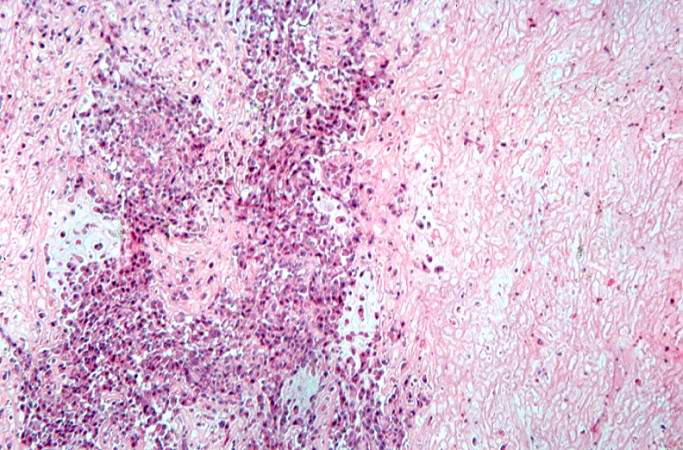
Pathological Findings
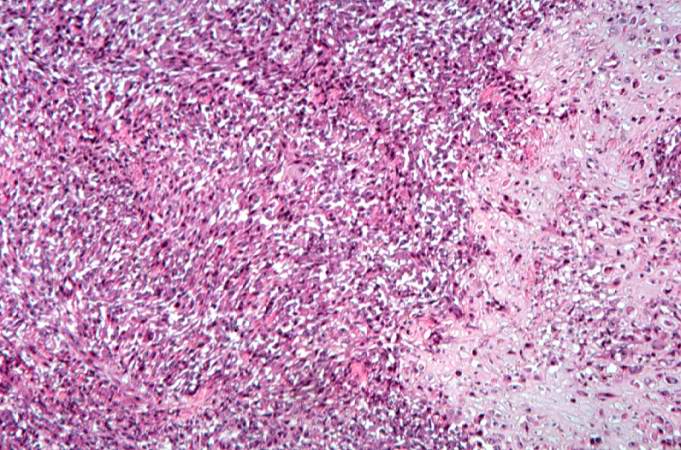
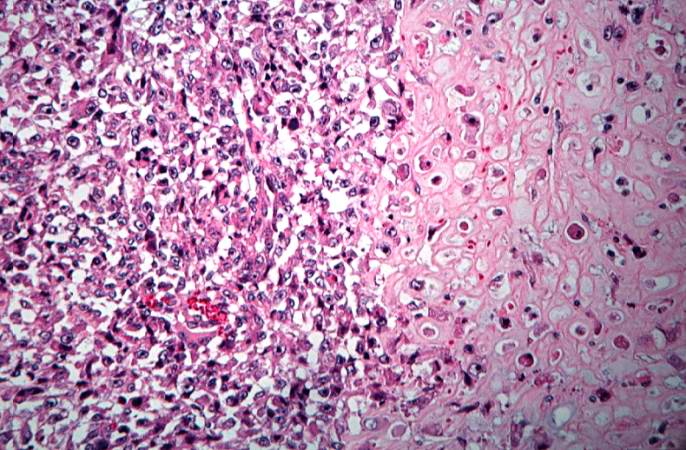
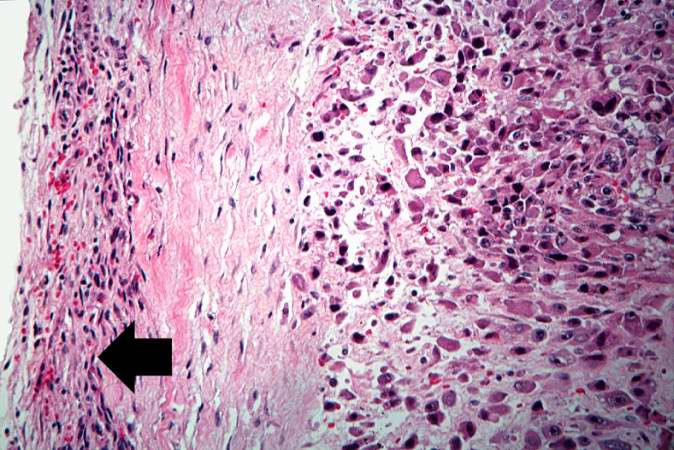
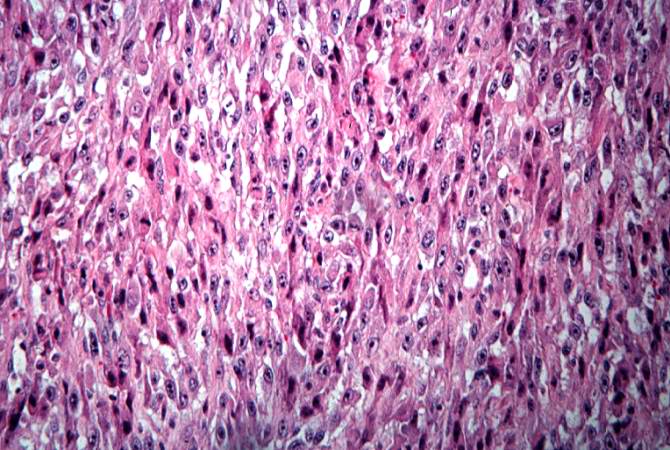
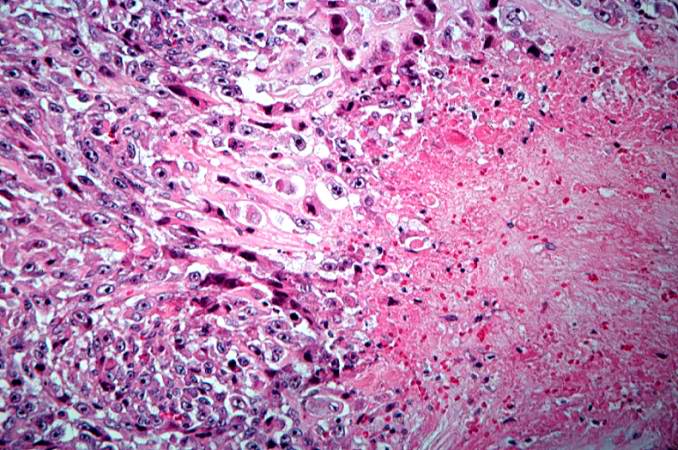
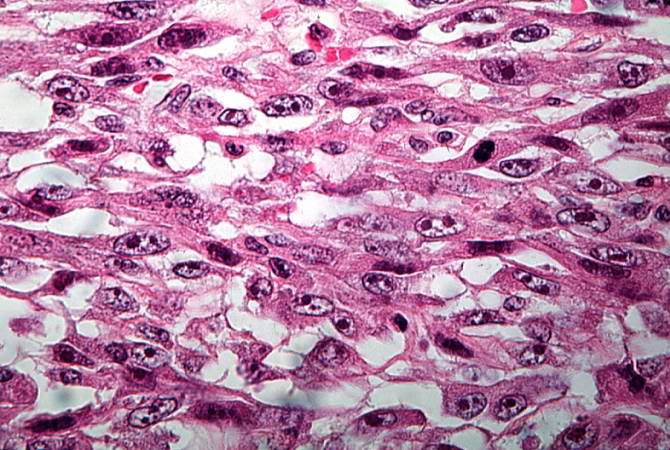
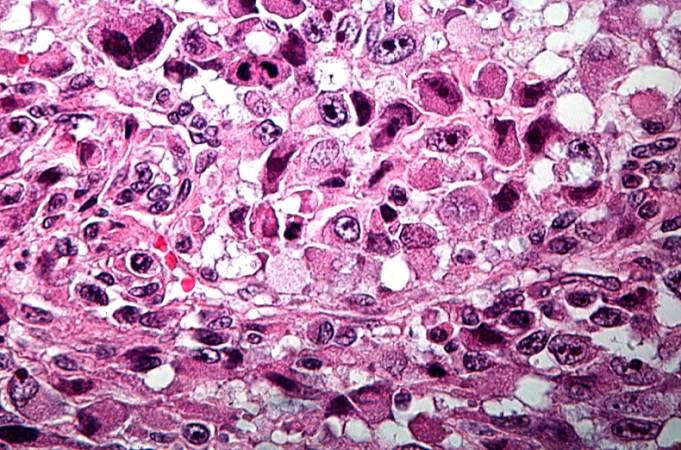
References
Additional Resources
- James, H. (1979). Promises in the Dark. New York: Bantam Books. ISBN 0-553-13453-1. Story of a young girl's osteosarcoma fight and its effect on her relationship with her boyfriend
- Trottier, Maxine (2005). Terry Fox: A Story of Hope. Markham, Ont: Scholastic Canada. ISBN 0-439-94888-6. About Terry Fox and his quest to raise $25 million for cancer research by running across Canada on his prosthetic leg. Also The Terry Fox Story, a 1983 movie.
- Belshaw, Sheila M. (2001). Fly With a Miracle. Denor Press. ISBN 0 9526056 7 8.
External links
- Superior Survivial Seen with Osteosarcoma 2004
- Osteosarcoma: A Multidisciplinary Approach to Diagnosis and Treatment
- http://www.emedicine.com/PED/topic1684.htm
- http://www.mayoclinic.org/osteosarcoma/index.html
- Support Group and Information for people with osteosarcoma
- Treatment Information from U.S. National Cancer Institute
- Osteosarcoma by Peter Buecker, MD and Mark Gebhardt, MD
- Green Drakkoman Foundation to assist Warriors of Rare Childhood Cancers
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson M.S., M.D.
Template:Osseous and chondromatous tumors de:Osteosarkom it:Osteosarcoma nl:Osteosarcoom