Orlistat: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 188: | Line 188: | ||
In clinical trials with XENICAL in adolescent patients ages 12 to 16 years, the profile of adverse reactions was generally similar to that observed in adults. | In clinical trials with XENICAL in adolescent patients ages 12 to 16 years, the profile of adverse reactions was generally similar to that observed in adults. | ||
|postmarketing=The following adverse reactions have been identified during postapproval use of XENICAL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to XENICAL exposure. | |||
* Rare cases of increase in transaminases and in alkaline phosphatase and hepatitis that may be serious have been reported. There have been reports of hepatic failure observed with the use of XENICAL in postmarketing surveillance, with some of these cases resulting in liver transplant or death. | |||
* Cases of reduced concentrations of cyclosporine have been reported when cyclosporine was co-administered with XENICAL. | |||
* Rare cases of hypersensitivity have been reported with the use of XENICAL. Signs and symptoms have included pruritus, rash, urticaria, angioedema, bronchospasm and anaphylaxis. Very rare cases of bullous eruption have been reported. | |||
* Reports of decreased prothrombin, increased INR and unbalanced anticoagulant treatment resulting in change of hemostatic parameters have been reported in patients treated concomitantly with XENICAL and anticoagulants. | |||
* Hypothyroidism has been reported in patients treated concomitantly with XENICAL and levothyroxine. | |||
=====Drug 1===== | * Acute oxalate nephropathy after treatment with XENICAL has been reported in patients with or at risk for renal disease. | ||
* Pancreatitis has been reported with the use of XENICAL in postmarketing surveillance. No causal relationship or physiopathological mechanism between pancreatitis and obesity therapy has been definitively established. | |||
* Lower gastrointestinal bleeding has been reported in patients treated with XENICAL. Most reports are nonserious; severe or persistent cases should be investigated further. | |||
* Convulsions have been reported in patients treated concomitantly with orlistat and antiepileptic drugs. | |||
|drugInteractions======Drug 1===== | |||
(Description) | (Description) | ||
Revision as of 22:56, 22 December 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Orlistat is a {{{drugClass}}} that is FDA approved for the treatment of a list of indications, separated by commas.. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- XENICAL is indicated for obesity management including weight loss and weight maintenance when used in conjunction with a reduced-calorie diet. XENICAL is also indicated to reduce the risk for weight regain after prior weight loss. XENICAL is indicated for obese patients with an initial body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 in the presence of other risk factors (e.g., hypertension, diabetes, dyslipidemia).
- TABLE 1 illustrates body mass index (BMI) according to a variety of weights and heights. The BMI is calculated by dividing weight in kilograms by height in meters squared. For example, a person who weighs 180 lbs and is 5'5" would have a BMI of 30.

Dosing
- The recommended dose of XENICAL is one 120-mg capsule three times a day with each main meal containing fat (during or up to 1 hour after the meal).
- The patient should be on a nutritionally balanced, reduced-calorie diet that contains approximately 30% of calories from fat. The daily intake of fat, carbohydrate, and protein should be distributed over three main meals. If a meal is occasionally missed or contains no fat, the dose of XENICAL can be omitted.
- Because XENICAL has been shown to reduce the absorption of some fat-soluble vitamins and beta-carotene, patients should be counseled to take a multivitamin containing fat-soluble vitamins to ensure adequate nutrition. The vitamin supplement should be taken at least 2 hours before or after the administration of XENICAL, such as at bedtime.
- For patients receiving both XENICAL and cyclosporine therapy, administer cyclosporine 3 hours after XENICAL.
- For patients receiving both XENICAL and levothyroxine therapy, administer levothyroxine and XENICAL at least 4 hours apart. Patients treated concomitantly with XENICAL and levothyroxine should be monitored for changes in thyroid function.
- Doses above 120 mg three times a day have not been shown to provide additional benefit.
- Based on fecal fat measurements, the effect of XENICAL is seen as soon as 24 to 48 hours after dosing. Upon discontinuation of therapy, fecal fat content usually returns to pretreatment levels within 48 to 72 hours.
DOSAGE FORMS AND STRENGTHS
- XENICAL 120 mg turquoise capsules imprinted with ROCHE and XENICAL 120 in black ink.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Pregnancy
- Patients with chronic malabsorption syndrome
- Patients with cholestasis
- Patients with known hypersensitivity to XENICAL or to any component of this product
Warnings
Concomitant Drug and Vitamin Use
- Data from a XENICAL and cyclosporine drug interaction study indicate a reduction in cyclosporine plasma levels when XENICAL was coadministered with cyclosporine. Therefore, XENICAL and cyclosporine should not be simultaneously coadministered. To reduce the chance of a drug-drug interaction, cyclosporine should be taken at least 3 hours before or after XENICAL in patients taking both drugs. In addition, in those patients whose cyclosporine levels are being measured, more frequent monitoring should be considered.
- Patients should be strongly encouraged to take a multivitamin supplement that contains fat-soluble vitamins to ensure adequate nutrition because XENICAL has been shown to reduce the absorption of some fat-soluble vitamins and beta-carotene. In addition, the levels of vitamin D and beta-carotene may be low in obese patients compared with non-obese subjects. The supplement should be taken once a day at least 2 hours before or after the administration of XENICAL, such as at bedtime.
- TABLE 2 illustrates the percentage of adult patients on XENICAL and placebo who developed a low vitamin level on two or more consecutive visits during 1 and 2 years of therapy in studies in which patients were not previously receiving vitamin supplementation.

- TABLE 3 illustrates the percentage of adolescent patients on XENICAL and placebo who developed a low vitamin level on two or more consecutive visits during the 1-year study.

- Weight-loss may affect glycemic control in patients with diabetes mellitus. A reduction in dose of oral hypoglycemic medication (e.g., sulfonylureas) or insulin may be required in some patients
Liver Injury
- There have been rare postmarketing reports of severe liver injury with hepatocellular necrosis or acute hepatic failure in patients treated with XENICAL, with some of these cases resulting in liver transplant or death. Patients should be instructed to report any symptoms of hepatic dysfunction (anorexia, pruritus, jaundice, dark urine, light-colored stools, or right upper quadrant pain) while taking XENICAL. When these symptoms occur, XENICAL and other suspect medications should be discontinued immediately and liver function tests and ALT and AST levels obtained.
Increases in Urinary Oxalate
- Some patients may develop increased levels of urinary oxalate following treatment with XENICAL. Cases of oxalate nephrolithiasis and oxalate nephropathy with renal failure have been reported. Monitor renal function when prescribing XENICAL to patients at risk for renal impairment and use with caution in those with a history of hyperoxaluria or calcium oxalate nephrolithiasis.
Cholelithiasis
Substantial weight loss can increase the risk of cholelithiasis. In a clinical trial of XENICAL for the prevention of type 2 diabetes, the rates of cholelithiasis as an adverse event were 2.9% (47/1649) for patients randomized to XENICAL and 1.8% (30/1655) for patients randomized to placebo.
Miscellaneous
- Organic causes of obesity (e.g., hypothyroidism) should be excluded before prescribing XENICAL.
- Patients should be advised to adhere to dietary guidelines. Gastrointestinal events may increase when XENICAL is taken with a diet high in fat (>30% total daily calories from fat). The daily intake of fat should be distributed over three main meals. If XENICAL is taken with any one meal very high in fat, the possibility of gastrointestinal effects increases.
Adverse Reactions
Clinical Trials Experience
Clinical Trials
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in patients.
- Commonly Observed (based on first year and second year data)
- Gastrointestinal (GI) symptoms were the most commonly observed treatment-emergent adverse events associated with the use of XENICAL in the seven double-blind, placebo-controlled clinical trials and are primarily a manifestation of the mechanism of action. (Commonly observed is defined as an incidence of ≥5% and an incidence in the XENICAL 120 mg group that is at least twice that of placebo.)

In general, the first occurrence of these events was within 3 months of starting therapy. Overall, approximately 50% of all episodes of GI adverse events associated with XENICAL treatment lasted for less than 1 week, and a majority lasted for no more than 4 weeks. However, GI adverse events may occur in some individuals over a period of 6 months or longer.
Discontinuation of Treatment
In controlled clinical trials, 8.8% of patients treated with XENICAL discontinued treatment due to adverse events, compared with 5.0% of placebo-treated patients. For XENICAL, the most common adverse events resulting in discontinuation of treatment were gastrointestinal.
Other Adverse Clinical Events
The following table lists other treatment-emergent adverse events from seven multicenter, double-blind, placebo-controlled clinical trials that occurred at a frequency of ≥2% among patients treated with XENICAL 120 mg three times a day and with an incidence that was greater than placebo during year 1 and year 2, regardless of relationship to study medication.

In the 4-year XENDOS study, the general pattern of adverse events was similar to that reported for the 1- and 2-year studies with the total incidence of gastrointestinal-related adverse events occurring in year 1 decreasing each year over the 4-year period.
In clinical trials in obese diabetic patients, hypoglycemia and abdominal distension were also observed.
Pediatric Patients
In clinical trials with XENICAL in adolescent patients ages 12 to 16 years, the profile of adverse reactions was generally similar to that observed in adults.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of XENICAL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to XENICAL exposure.
- Rare cases of increase in transaminases and in alkaline phosphatase and hepatitis that may be serious have been reported. There have been reports of hepatic failure observed with the use of XENICAL in postmarketing surveillance, with some of these cases resulting in liver transplant or death.
- Cases of reduced concentrations of cyclosporine have been reported when cyclosporine was co-administered with XENICAL.
- Rare cases of hypersensitivity have been reported with the use of XENICAL. Signs and symptoms have included pruritus, rash, urticaria, angioedema, bronchospasm and anaphylaxis. Very rare cases of bullous eruption have been reported.
- Reports of decreased prothrombin, increased INR and unbalanced anticoagulant treatment resulting in change of hemostatic parameters have been reported in patients treated concomitantly with XENICAL and anticoagulants.
- Hypothyroidism has been reported in patients treated concomitantly with XENICAL and levothyroxine.
- Acute oxalate nephropathy after treatment with XENICAL has been reported in patients with or at risk for renal disease.
- Pancreatitis has been reported with the use of XENICAL in postmarketing surveillance. No causal relationship or physiopathological mechanism between pancreatitis and obesity therapy has been definitively established.
- Lower gastrointestinal bleeding has been reported in patients treated with XENICAL. Most reports are nonserious; severe or persistent cases should be investigated further.
- Convulsions have been reported in patients treated concomitantly with orlistat and antiepileptic drugs.
Drug Interactions
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
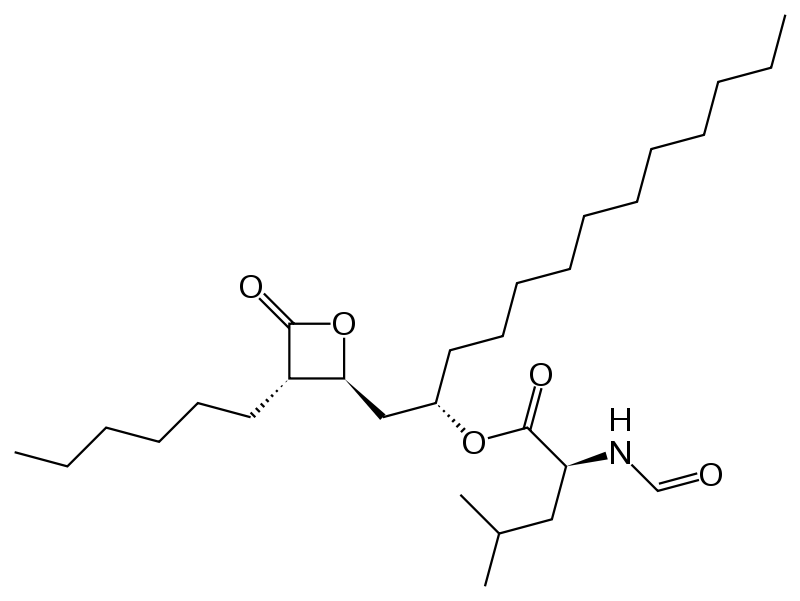
| |
Orlistat
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Orlistat Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Orlistat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Orlistat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Orlistat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Orlistat Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.