Organocatalysis: Difference between revisions
No edit summary |
(No difference)
|
Revision as of 15:06, 10 June 2009
|
WikiDoc Resources for Organocatalysis |
|
Articles |
|---|
|
Most recent articles on Organocatalysis Most cited articles on Organocatalysis |
|
Media |
|
Powerpoint slides on Organocatalysis |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Organocatalysis at Clinical Trials.gov Trial results on Organocatalysis Clinical Trials on Organocatalysis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Organocatalysis NICE Guidance on Organocatalysis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Organocatalysis Discussion groups on Organocatalysis Patient Handouts on Organocatalysis Directions to Hospitals Treating Organocatalysis Risk calculators and risk factors for Organocatalysis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Organocatalysis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [5] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
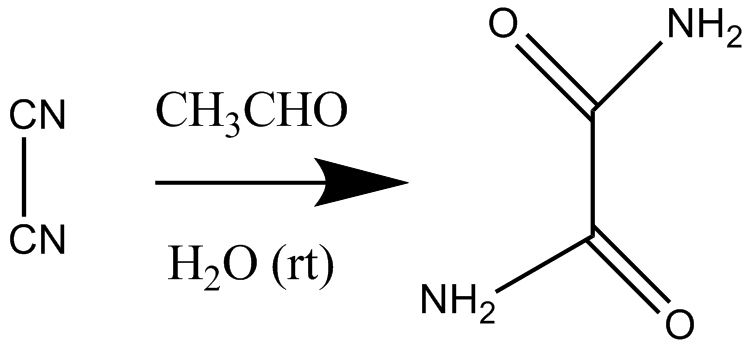
In organic chemistry, the term Organocatalysis (a concatenation of the terms "organic" and "catalyst") refers to a form of catalysis, whereby the rate of a chemical reaction is increased by an organic catalyst referred to as an "organocatalyst" consisting of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds [3] [4] [5] [6] [7] [8]. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.
The term "organocatalysis" was created by David MacMillan in 2000 from the old and well known concept of "organic catalysis" introduced by the German chemist Wolfgang Langenbeck; "organocatalysis" is nothing more than a new name for an old methodology, but thus gives fresh impulses for intensive research in the following years.
Organocatalysts which display secondary amine functionality can be described as performing either enamine catalysis (by forming catalytic quantities of an active enamine nucleophile) or iminium catalysis (by forming catalytic quantities of an activated iminium electrophile). This mechanism is typical for covalent organocatalysis. Covalent binding of substrate normally requires high catalyst loading (for proline-catalysis typically 20-30 mol%). Noncovalent interactions such as hydrogen-bonding facilitates low catalyst loadings (down to 0.001 mol%).
Two main advantages of organocatalysis are:
- there is no need for metal-based catalysis thus making a contribution to green chemistry. In this context, simple organic acids have been used as catalyst for the modification of cellulose in water on multi-ton scale.[9]
- when the organocatalyst is chiral an avenue is opened to asymmetric catalysis, for example the use of proline in aldol reactions,
Introduction
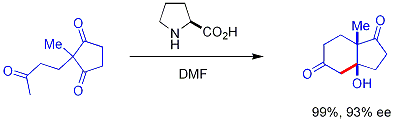
Regular achiral organocatalysts are based on nitrogen such as pyridine used in the Doebner modification of the Aldol condensation, DMAP used in esterfications and DABCO used in the Baylis-Hillman reaction. Thiazolium salts are employed in the Stetter reaction. These catalysts and reactions have a long history but current interest in organocatalysis is focused on asymmetric catalysis with chiral catalysts and this particular branch is called asymmetric organocatalysis or enantioselective organocatalysis . A pioneering reaction developed in the 1970s by teams of Hoffmann-La Roche[10] [11] and Schering AG[12] that sums it all up is the Hajos-Parrish-Eder-Sauer-Wiechert reaction:
In this reaction, naturally occurring chiral proline is the chiral catalyst in an Aldol reaction. The starting material is an achiral triketone and it requires just 3% of proline to obtain the reaction product, a ketol in 93% enantiomeric excess. Discovered in the 1970's the original Hajos-Parrish catalytic procedure shown in the reaction equation leading to the optically active bicyclic ketol as well as the Eder-Sauer-Wiechert modification leading to the optically active dione paved the way of asymmetric organocatalysis.
Hajos and Parrish worked at ambient temperature using a catalytic amount (3% molar equiv.) of (S)-(-)-proline enabling them to isolate the optically active intermediate bicyclic ketol shown above. The Schering group used non biological conditions using (S)-Proline (47 mol%), 1N perchloric acid, in acetonitrile at 80 °C. Hence, they could not isolate the Hajos, Parrish intermediate bicyclic ketols but instead the enedione condensation product.
The asymmetric synthesis of the Wieland-Miescher ketone (1985) is also based on proline and another early application was one of the transformations in the total synthesis of Erythromycin by Robert B. Woodward (1981) [13].
Many chiral organocatalysts are an adaptation of chiral ligands (which together with a metal center also catalyze asymmetric reactions) and both concepts overlap to some degree.
Organocatalyst classes
Organocatalysts for asymmetric synthesis can be grouped in several classes:
- Biomolecules: notably proline, phenylalanine, the cinchona alkaloids, certain oligopeptides.
- Synthetic catalysts derived from biomolecules. Examples of proline derivatives are MacMillan Imidazolidinones and the CBS catalyst
- TADDOLS
- Derivatives of BINOL such as NOBIN
- Triazolium salts as next-generation Stetter reaction catalysts
- Organocatalysts based on thioureas
Examples of asymmetric reactions involving organocatalysts are:
- Asymmetric Diels-Alder reactions
- Asymmetric Michael reactions
- Asymmetric Mannich reactions
- Shi epoxidation
- Organocatalytic transfer hydrogenation
Imidazolidinone organocatalysis
A certain class of imidazolidinone compounds (also called MacMillan organocatalysts) are suitable catalysts for many asymmetric reactions such as asymmetric DA reactions. The original such compound was derived from the biomolecule phenylalanine in two chemical steps (amidation with methylamine followed by condensation reaction with acetone) which leave the chirality intact [14]:
This catalyst works by forming a iminium ion with carbonyl groups of α,β-unsaturated aldehydes (enals) and enones in a rapid chemical equilibrium. This iminium activation is similar to activation of carbonyl groups by a Lewis acid and both catalysts lower the substrates LUMO [15]:
The transient iminium intermediate is chiral which is transferred to the reaction product via chiral induction. The catalysts have been used in Diels-Alder reactions, Michael additions, Friedel-Crafts alkylations, transfer hydrogenations and epoxidations.
One example is the asymmetric synthesis of the drug warfarin (in equilibrium with the hemiketal) in a Michael addition of 4-hydroxycoumarin and benzylideneacetone [16]:
A recent exploit is the vinyl alkylation of crotonaldehyde with an organotrifluoroborate salt [17]:
For other examples of its use: see organocatalytic transfer hydrogenation and asymmetric DA reactions.
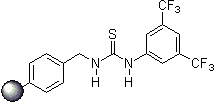
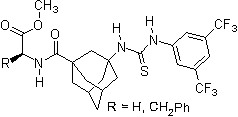
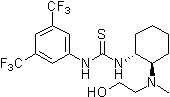
Thiourea organocatalysis
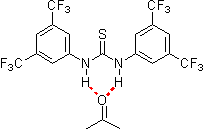
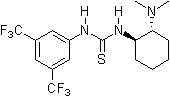
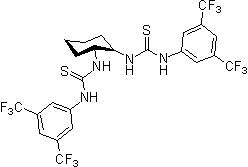
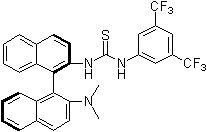
In nature noncovalent interactions such as hydrogen bonding ("partial protonation") play a crucial role in enzyme catalysis that is characterized by selective substrate recognition (molecular recognition), substrate activation, and enormous acceleration of organic transformations. Based on the pioneering examinations by Kelly, Etter, Jorgensen, Hine, Curran, Göbel, and De Mendoza (see review articles cited below) on hydrogen bonding interactions of small, metal-free compounds with electron-rich binding sites Schreiner and co-workers performed series of theoretical and experimental systematic investigations towards the hydrogen-bonding ability of various thiourea derivatives [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31]. This purely organic compounds revealed effective acceleration of simple Diels-Alder reaction, act like weak Lewis acid catalyst, but act through explicit double hydrogen bonding instead of covalent binding known from traditional metal-ion mediated catalysis. Schreiner and co-workers identified and indroduced electron-poor thiourea derivatives as hydrogen-bonding organocatalysts. N,N'-bis[[3,5-bis(trifluormethyl)phenyl thiourea is to date the most effective achiral thiourea derivative and combines all typical structural features for double H-bonding mediated organocatalysis:
- electron-poor
- rigid structure
- non-coordinating, electron withdrawing substituents in 3,4, and/or 5 position of a phenyl ring
- the trifluoromethyl-group is the preferred substituent
Advantages of thiourea derivatives:
- no product inhibition due to weak enthalpic binding, but specific binding-“recognition“
- low catalyst-loading (down to 0.001 mol%)[citation needed]
- high TOF values (up to 2,000 h–1)[citation needed]
- simple and inexpensive synthesis
- easily to modulate and to handle, no inert atmosphere necessary
- immobilization on solid phase (polymer-bound organocatalysts), catalyst recovery and reusability
- catalysis under almost neutral conditions (pka thiourea 21.0), acid-sensitive substrates are tolerated
- metal-free, not toxic (compare traditional metal-containing Lewis-acid catalysts
- water-tolerant, even catalytically effective in water or aqueous media
- environmentally benign ("Green Chemistry")
To date various organic transformations are organocatalyzed through hydrogen-bonding N,N'-bis[[3,5-bis(trifluormethyl)phenyl thiourea at low catalyst loadings and in good to excellent product yields. This electron-poor thiourea derivative has proven to be the benchmark for noncovalent organocatalysis utilizing explicit hydrogen-bonding as well as to be the basis for development of a wide range of catalytically active derivatives.
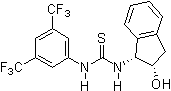
Since 2001 research groups world-wide (e.g., Berkessel, Connon, Jacobsen, Nagaswa, Takemoto) have realized the potential of thiourea derivatives and developed various achiral/chiral mono- and bifunctional derivatives incorporating the electron-poor 3,5-bis(trifluoromethyl)phenyl substrate-"anchor" functionality. Meanwhile a broad spectrum of organic transformations are performed through hydrogen-bonding organocatalysis and the research ist still in the focus of interest.
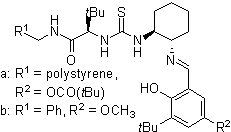 |
 |
 |
 |
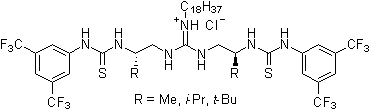 |
 |
 |
 |
 |
 |
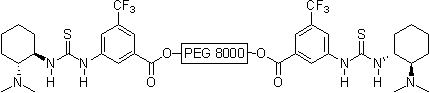 |
 |
 |
 |
References
- ↑ Justus von Liebig (1860). "Ueber die Bildung des Oxamids aus Cyan". Annalen der Chemie und Pharmacie. 113 (2): 246–247. doi:10.1002/jlac.18601130213.
- ↑ W. Langenbeck, Liebigs Ann. 1929, 469, 16.
- ↑ Berkessel, A., Groeger, H. (2005). Asymmetric Organocatalysis. Weinheim: Wiley-VCH. ISBN 3-527-30517-3.
- ↑ Special Issue: List, Benjamin (2007). "Organocatalysis". Chem. Rev. 107 (12): 5413–5883. doi:10.1021/cr078412e.
- ↑ Peter I. Dalko, Lionel Moisan, review: "In the Golden Age of Organocatalysis", Angew. Chem. Int. Ed. 2004, 43, 5138–5175
- ↑ Matthew J. Gaunt, Carin C.C. Johansson, Andy McNally, Ngoc T. Vo, review: "Enantioselective organocatalysis" Drug Discovery Today, 2007, 12(1/2), 8-27
- ↑ Dieter Enders, Christoph Grondal, Matthias R. M. Hüttl, review: "Asymmetric Organocatalytic Domino Reactions", Angew. Chem. Int. Ed. 2007, 46, 1570–1581
- ↑ Enantioselective Organocatalysis Peter I. Dalko and Lionel Moisan Angew. Chem. Int. Ed. 2001, 40, 3726 ± 3748
- ↑ International Patent WO 2006068611 A1 20060629 “ Direct Homogeneous and Heterogeneous Organic Acid and Amino Acid-Catalyzed Modification of Amines and Alcohols” Inventors: Armando Córdova, Stockholm, Sweden; Jonas Hafrén, Stockholm, Sweden.
- ↑ Z. G. Hajos, D. R. Parrish, German Patent DE 2102623 1971
- ↑ Asymmetric synthesis of bicyclic intermediates of natural product chemistry Zoltan G. Hajos, David R. Parrish J. Org. Chem.; 1974; 39(12); 1615-1621. doi:10.1021/jo00925a003
- ↑ New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures Angewandte Chemie International Edition in English Volume 10, Issue 7, Date: July 1971, Pages: 496-497 Ulrich Eder, Gerhard Sauer, Rudolf Wiechert doi:10.1002/anie.197104961
- ↑ Asymmetric total synthesis of erythromcin. 1. Synthesis of an erythronolide A secoacid derivative via asymmetric induction R. B. Woodward, E. Logusch, K. P. Nambiar, K. Sakan, D. E. Ward, B. W. Au-Yeung, P. Balaram, L. J. Browne, P. J. Card, C. H. Chen J. Am. Chem. Soc.; 1981; 103(11); 3210-3213. doi:10.1021/ja00401a049
- ↑ New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels-Alder Reaction Ahrendt, K. A.; Borths, C. J.; MacMillan, D. W. C. J. Am. Chem. Soc.; (Communication); 2000; 122(17); 4243-4244. doi:10.1021/ja000092s
- ↑ Modern Strategies in Organic Catalysis: The Advent and Development of Iminium Activation Gérald Lelais and David W. C. MacMillan VOL. 39, NO. 3, 79 • 2006 Aldrichimica Acta http://www.sigmaaldrich.com/aldrich/brochure/al_acta_39_3.pdf
- ↑ Organocatalytic Asymmetric Michael Reaction of Cyclic 1,3-Dicarbonyl Compounds and ,-Unsaturated Ketones - A Highly Atom-Economic Catalytic One-Step Formation of Optically Active Warfarin Anticoagulant Angewandte Chemie International EditionVolume 42, Issue 40, Date: October 20, 2003, Pages: 4955-4957 Nis Halland, Tore Hansen, Karl Anker Jørgensen doi:10.1002/anie.200352136
- ↑ Organocatalytic Vinyl and Friedel-Crafts Alkylations with Trifluoroborate Salts Sandra Lee and David W. C. MacMillan J. AM. CHEM. SOC. 2007, 129, 15438-15439 doi:10.1021/ja0767480
- ↑ Alexander Wittkopp, Peter R. Schreiner, "Diels-Alder Reactions in Water and in Hydrogen-Bonding Environments", book chapter in "The Chemistry of Dienes and Polyenes" Zvi Rappoport (Ed.), Volume 2, John Wiley & Sons Inc.; Chichester, 2000, 1029-1088. ISBN 0-471-72054-2.
- ↑ Alexander Wittkopp, "Organocatalysis of Diels-Alder Reactions by Neutral Hydrogen Bond Donors in Organic and Aqueous Solvents", dissertation written in German, Universität Göttingen, 2001. english abstract/download: [1]
- ↑ P. R. Schreiner and A. Wittkopp (2002). "H-Bonding Additives Act Like Lewis Acid Catalysts". Org. Lett. 4 (2): 217–220. doi:10.1021/ol017117s.
- ↑ A. Wittkopp and P. R. Schreiner (2003). "Metal-Free, Noncovalent Catalysis of Diels-Alder Reactions by Neutral Hydrogen Bond Donors in Organic Solvents and in Water". Chemistry - A European Journal. 9 (2): 407–414. doi:10.1002/chem.200390042.
- ↑ Peter R. Schreiner, review: "Metal-free organocatalysis through explicit hydrogen bonding interactions", Chem. Soc. Rev. 2003, 32, 289-296. abstract/download:[2]
- ↑ M. Kotke and P. R. Schreiner (2006). "Acid-free, organocatalytic acetalization". Tetrahedron. 62 (2–3): 434–439. doi:10.1016/j.tet.2005.09.079.
- ↑ Christian M. Kleiner, Peter R. Schreiner, "Hydrophobic amplification of noncovalent organocatalysis", Chem. Commun. 2006, 4315-4017.abstract/download:[3]
- ↑ M. Kotke and P. Schreiner (2007). "Generally Applicable Organocatalytic Tetrahydropyranylation of Hydroxy Functionalities with Very Low Catalyst Loading". Synthesis. 2007 (5): 779–790. doi:10.1055/s-2007-965917.
- ↑ L. Wanka and C. Cabrele (2007). "γ-Aminoadamantanecarboxylic Acids Through Direct C-H Bond Amidations". European Journal of Organic Chemistry. 2007 (9): 1474–1490. doi:10.1002/ejoc.200600975.
- ↑ Z. Zhang and P. R. Schreiner (2007). "Thiourea-Catalyzed Transfer Hydrogenation of Aldimines". Synlett. 2007 (9): 1455–1457. doi:10.1055/s-2007-980349.
- ↑ M. P. Petri (2004). "Activation of Carbonyl Compounds by Double Hydrogen Bonding: An Emerging Tool in Asymmetric Catalysis". Angewandte Chemie International Edition. 43 (16): 2062–2064. doi:10.1002/anie.200301732.
- ↑ Yoshiji Takemoto, review: "Recognition and activation by ureas and thioureas: stereoselective reactions using ureas and thioureas as hydrogen-bonding donors", Org. Biomol. Chem. 2005, 3, 4299-4306. abstract/download: [4]
- ↑ Mark S. Taylor, Eric N. Jacobsen (2006). "Asymmetric Catalysis by Chiral Hydrogen-Bond Donors". Angewandte Chemie International Edition. 45 (10): 1520–1543. doi:10.1002/anie.200503132.
- ↑ J. C. Stephen (2006). "Organocatalysis Mediated by (Thio)urea Derivatives". Chemistry - A European Journal. 12 (21): 5418–5427. doi:10.1002/chem.200501076.