Oprelvekin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Allergic Reactions Including Anaphylaxis
See full prescribing information for complete Boxed Warning.
Condition Name:Oprelvekin has caused allergic or hypersensitivity reactions, including anaphylaxis. Administration of Oprelvekin should be permanently discontinued in any patient who develops an allergic or hypersensitivity reaction
|
Overview
Oprelvekin is a thrombopoietic growth factor that is FDA approved for the prophylaxis of severe thrombocytopenia and the reduction of the need for platelet transfusions following myelosuppressive chemotherapy in adult patients with nonmyeloid malignancies who are at high risk of severe thrombocytopenia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, candidiasis, nausea, oral candidiasis, vomiting, dizziness, fatigue, headache, blurred vision, conjunctival hyperemia and dyspnea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- The recommended dose of Neumega in adults without severe renal impairment is 50 mcg/kg given once daily. Neumega should be administered subcutaneously as a single injection in either the abdomen, thigh, or hip (or upper arm if not self-injecting). A safe and effective dose has not been established in children.
- The recommended dose of Neumega in adults with severe renal impairment (creatinine clearance <30 mL/min) is 25 mcg/kg. An estimate of the patient's creatinine clearance (CLcr) in mL/min is required. CLcr in mL/min may be estimated from a spot serum creatinine (mg/dL) determination using the following formula:
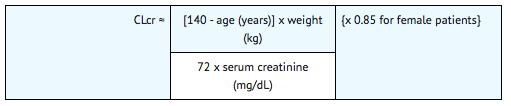
- Dosing should be initiated six to 24 hours after the completion of chemotherapy. Platelet counts should be monitored periodically to assess the optimal duration of therapy. Dosing should be continued until the post-nadir platelet count is ≥50,000/μL. In controlled clinical trials, doses were administered in courses of 10 to 21 days. Dosing beyond 21 days per treatment course is not recommended.
- Treatment with Neumega should be discontinued at least two days before starting the next planned cycle of chemotherapy.
Preparation of Neumega
- Neumega is a sterile, white, preservative-free, lyophilized powder for subcutaneous injection upon reconstitution. Reconstitute the Neumega 5 mg vial using the 1.0 mL of Sterile Water for Injection, USP (without preservative) contained in the pre-filled syringe included in the kit. The reconstituted Neumega solution is clear, colorless, isotonic, with a pH of 7.0, and contains 5 mg/mL of Neumega. Any unused portion of the reconstituted Neumega solution should be discarded.
- During reconstitution, the Sterile Water for Injection, USP should be directed at the side of the vial and the contents gently swirled. Excessive or vigorous agitation should be avoided.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulate matter is present or the solution is discolored, the vial should not be used.
- Administer Neumega within 3 hours following reconstitution. Reconstituted Neumega may be refrigerated [2°C to 8°C (36°F to 46°F)] or maintained at room temperature [up to 25°C (77°F)]. Do not freeze or shake the reconstituted solution.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Oprelvekin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Oprelvekin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Oprelvekin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Oprelvekin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Oprelvekin in pediatric patients.
Contraindications
- Oprelvekin is contraindicated in patients with a history of hypersensitivity to Oprelvekin or any component of the product.
Warnings
|
Allergic Reactions Including Anaphylaxis
See full prescribing information for complete Boxed Warning.
Condition Name:Oprelvekin has caused allergic or hypersensitivity reactions, including anaphylaxis. Administration of Oprelvekin should be permanently discontinued in any patient who develops an allergic or hypersensitivity reaction
|
Allergic Reactions Including Anaphylaxis
- In the post-marketing setting, Oprelvekin has caused allergic or hypersensitivity reactions, including anaphylaxis. The administration of Oprelvekin should be attended by appropriate precautions in case allergic reactions occur. In addition, patients should be counseled about the symptoms for which they should seek medical attention. Signs and symptoms reported included edema of the face, tongue, or larynx; shortness of breath; wheezing; chest pain; hypotension (including shock); dysarthria; loss of consciousness; mental status changes; rash; urticaria; flushing and fever. Reactions occurred after the first dose or subsequent doses of Oprelvekin. Administration of Oprelvekin should be permanently discontinued in any patient who develops an allergic or hypersensitivity reaction.
Increased Toxicity Following Myeloablative Therapy
- Oprelvekin is not indicated following myeloablative chemotherapy. In a randomized, placebo-controlled Phase 2 study, the effectiveness of Oprelvekin was not demonstrated. In this study, a statistically significant increased incidence in edema, conjunctival bleeding, hypotension, and tachycardia was observed in patients receiving Oprelvekin as compared to placebo.
- The following severe or fatal adverse reactions have been reported in post-marketing use in patients who received Oprelvekin following bone marrow transplantation: fluid retention or overload (eg, facial edema, pulmonary edema), capillary leak syndrome, pleural and pericardial effusion, papilledema and renal failure.
Fluid Retention
- Oprelvekin is known to cause serious fluid retention that can result in peripheral edema, dyspnea on exertion, pulmonary edema, capillary leak syndrome, atrial arrhythmias, and exacerbation of pre-existing pleural effusions. Severe fluid retention, some cases resulting in death, was reported following recent bone marrow transplantation in patients who have received Oprelvekin. Oprelvekin is not indicated following myeloablative chemotherapy. It should be used with caution in patients with clinically evident congestive heart failure, patients who may be susceptible to developing congestive heart failure, patients receiving aggressive hydration, patients with a history of heart failure who are well-compensated and receiving appropriate medical therapy, and patients who may develop fluid retention as a result of associated medical conditions or whose medical condition may be exacerbated by fluid retention.
- Fluid retention is reversible within several days following discontinuation of Oprelvekin During dosing with Oprelvekin fluid balance should be monitored and appropriate medical management is advised.
- Close monitoring of fluid and electrolyte status should be performed in patients receiving chronic diuretic therapy. Sudden deaths have occurred in oprelvekin-treated patients receiving chronic diuretic therapy and ifosfamide who developed severe hypokalemia.
- Pre-existing fluid collections, including pericardial effusions or ascites, should be monitored. Drainage should be considered if medically indicated.
Dilutional Anemia
- Moderate decreases in hemoglobin concentration, hematocrit, and red blood cell count (~10% to 15%) without a decrease in red blood cell mass have been observed. These changes are predominantly due to an increase in plasma volume (dilutional anemia) that is primarily related to renal sodium and water retention. The decrease in hemoglobin concentration typically begins within three to five days of the initiation of Oprelvekin, and is reversible over approximately a week following discontinuation of Oprelvekin.
Cardiovascular Events
- Oprelvekin use is associated with cardiovascular events including arrhythmias and pulmonary edema. Cardiac arrest has been reported, but the causal relationship to Oprelvekin is uncertain. Use with caution in patients with a history of atrial arrhythmias, and only after consideration of the potential risks in relation to anticipated benefit. In clinical trials, cardiac events including atrial arrhythmias (atrial fibrillation or atrial flutter) occurred in 15% (23/157) of patients treated with Oprelvekin at doses of 50 mcg/kg. Arrhythmias were usually brief in duration; conversion to sinus rhythm typically occurred spontaneously or after rate-control drug therapy. Approximately one-half (11/24) of the patients who were rechallenged had recurrent atrial arrhythmias. Clinical sequelae, including stroke, have been reported in patients who experienced atrial arrhythmias while receiving Oprelvekin.
- The mechanism for induction of arrhythmias is not known. Oprelvekin was not directly arrhythmogenic in animal models. In some patients, development of atrial arrhythmias may be due to increased plasma volume associated with fluid retention.
- In the post-marketing setting, ventricular arrhythmias have been reported, generally occurring within two to seven days of initiation of treatment.
Nervous System Events
- Stroke has been reported in the setting of patients who develop atrial fibrillation/flutter while receiving Oprelvekin. Patients with a history of stroke or transient ischemic attack may also be at increased risk for these events.
Papilledema
- Papilledema has been reported in 2% (10/405) of patients receiving Oprelvekin in clinical trials following repeated cycles of exposure. The incidence was higher, 16% (7/43) in children than in adults, 1% (3/362). Nonhuman primates treated with Oprelvekin at a dose of 1,000 mcg/kg SC once daily for four to 13 weeks developed papilledema that was not associated with inflammation or any other histologic abnormality and was reversible after dosing was discontinued. Oprelvekin should be used with caution in patients with pre-existing papilledema, or with tumors involving the central nervous system since it is possible that papilledema could worsen or develop during treatment. Changes in visual acuity and/or visual field defects ranging from blurred vision to blindness can occur in patients with papilledema taking Oprelvekin.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
- Three hundred twenty-four subjects, with ages ranging from eight months to 75 years, have been exposed to Oprelvekin treatment in clinical studies. Subjects have received up to six (eight in pediatric patients) sequential courses of Oprelvekin treatment, with each course lasting from one to 28 days. Apart from the sequelae of the underlying malignancy or cytotoxic chemotherapy, most adverse events were mild or moderate in severity and reversible after discontinuation of Oprelvekin dosing.
- In general, the incidence and type of adverse events were similar between Oprelvekin 50 mcg/kg and placebo groups. The most frequently reported serious adverse events were neutropenic fever, syncope, atrial fibrillation, fever and pneumonia. The most commonly reported adverse events were edema, dyspnea, tachycardia, conjunctival injection, palpitations, atrial arrhythmias, and pleural effusions. The most frequently reported adverse reactions resulting in clinical intervention (eg, discontinuation of Oprelvekin, adjustment in dosage, or the need for concomitant medication to treat an adverse reaction symptom) were atrial arrhythmias, syncope, dyspnea, congestive heart failure, and pulmonary edema. Selected adverse events that occurred in ≥10% of Oprelvekin-treated patients are listed in TABLE 3.
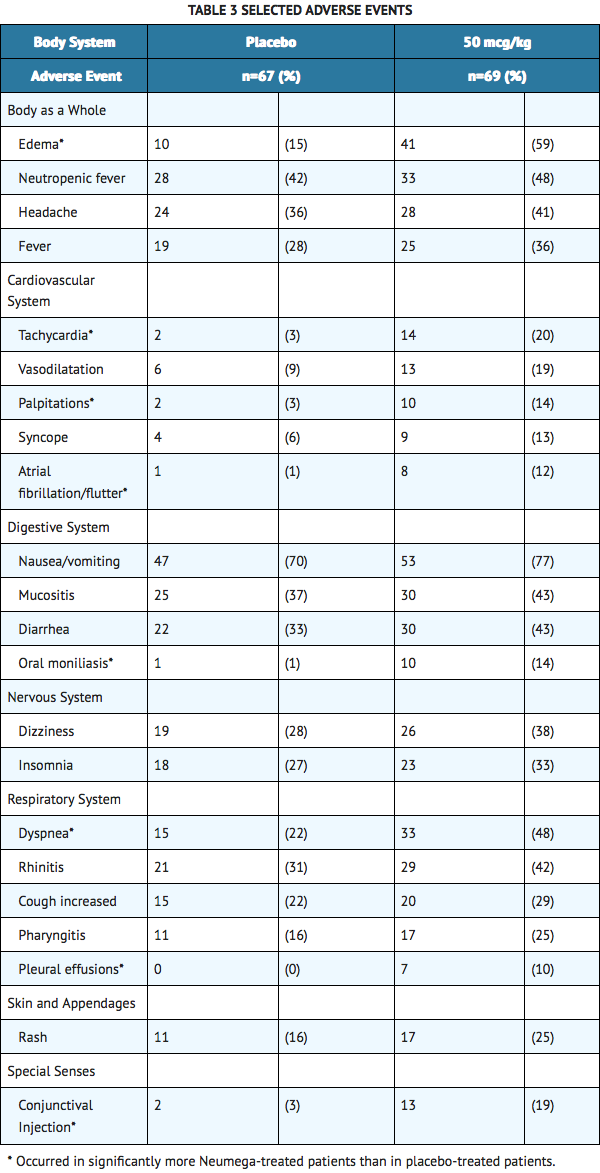
- The following adverse events also occurred more frequently in cancer patients receiving Oprelvekin than in those receiving placebo: blurred vision, paresthesia, dehydration, skin discoloration, exfoliative dermatitis, and eye hemorrhage. Other than a higher incidence of severe asthenia in Oprelvekin treated patients (10 [14%] in Oprelvekin patients versus two [3%] in placebo patients), the incidence of severe or life-threatening adverse events was comparable in the Oprelvekin and placebo treatment groups.
- Two patients with cancer treated with Oprelvekin experienced sudden death that the investigator considered possibly or probably related to Oprelvekin Both deaths occurred in patients with severe hypokalemia (<3.0 mEq/L) who had received high doses of ifosfamide and were receiving daily doses of a diuretic.
- Other serious events associated with Oprelvekin were papilledema and cardiovascular events including atrial arrhythmias and stroke. In addition, cardiomegaly was reported in children.
- The following adverse events, occurring in ≥10% of patients, were observed at equal or greater frequency in placebo-treated patients: asthenia, pain, chills, abdominal pain, infection, anorexia, constipation, dyspepsia, ecchymosis, myalgia, bone pain, nervousness, and alopecia. The incidence of fever, neutropenic fever, flu-like symptoms, thrombocytosis, thrombotic events, the average number of units of red blood cells transfused per patient, and the duration of neutropenia <500 cells/μL were similar in the Oprelvekin 50 mcg/kg and placebo groups.
Immunogenicity
- In clinical studies that evaluated the immunogenicity of Oprelvekin, two of 181 patients (1%) developed antibodies to Oprelvekin. In one of these two patients, neutralizing antibodies to Oprelvekin were detected in an unvalidated assay. The clinical relevance of the presence of these antibodies is unknown. In the post-marketing setting, cases of allergic reactions, including anaphylaxis have been reported. The presence of antibodies to Oprelvekin was not assessed in these patients.
- The data reflect the percentage of patients whose test results were considered positive for antibodies to Oprelvekin and are highly dependent on the sensitivity and specificity of the assay. Additionally the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, concomitant medications, and underlying disease. For these reasons, comparisons of the incidence of antibodies to Oprelvekin with incidence of antibodies to other products may be misleading.
Abnormal Laboratory Values
- The most common laboratory abnormality reported in patients in clinical trials was a decrease in hemoglobin concentration predominantly as a result of expansion of the plasma volume. The increase in plasma volume is also associated with a decrease in the serum concentration of albumin and several other proteins (eg, transferrin and gamma globulins). A parallel decrease in calcium without clinical effects has been documented.
- After daily SC injections, treatment with Oprelvekin resulted in a two-fold increase in plasma fibrinogen. Other acute-phase proteins also increased. These protein levels returned to normal after dosing with Oprelvekin was discontinued. Von Willebrand factor (vWF) concentrations increased with a normal multimer pattern in healthy subjects receiving Oprelvekin.
Postmarketing Experience
- Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reactions, (2) frequency of reporting, or (3) strength of causal connection to Oprelvekin.
- The following adverse reactions have been reported during the post-marketing use of Oprelvekin:
- allergic reactions and anaphylaxis/anaphylactoid reactions
- papilledema
- visual disturbances ranging from blurred vision to blindness
- optic neuropathy
- ventricular arrhythmias
- capillary leak syndrome
- renal failure
- injection site reactions (dermatitis, pain, and discoloration)
Drug Interactions
- Most patients in trials evaluating Oprelvekin were treated concomitantly with filgrastim (G-CSF) with no adverse effect of Oprelvekin on the activity of G-CSF. No information is available on the clinical use of sargramostim (GM-CSF) with Oprelvekin in human subjects. However, in a study in nonhuman primates in which Oprelvekin and GM-CSF were coadministered, there were no adverse interactions between Oprelvekin and GM-CSF and no apparent difference in the pharmacokinetic profile of Oprelvekin.
- Drug interactions between Oprelvekin and other drugs have not been fully evaluated. Based on in vitro and nonclinical in vivo evaluations of Oprelvekin, drug-drug interactions with known substrates of P450 enzymes would not be predicted.
Use in Specific Populations
Pregnancy
- Oprelvekin has been shown to have embryocidal effects in pregnant rats and rabbits when given in doses of 0.2 to 20 times the human dose. There are no adequate and well-controlled studies of Oprelvekin in pregnant women. Oprelvekin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Oprelvekin has been tested in studies of fertility, early embryonic development, and pre- and postnatal development in rats and in studies of organogenesis (teratogenicity) in rats and rabbits. Parental toxicity has been observed when Oprelvekin is given at doses of two to 20 times the human dose (≥100 mcg/kg/day) in the rat and at 0.02 to 2.0 times the human dose (≥1 mcg/kg/day) in the rabbit. Findings in pregnant rats consisted of transient hypoactivity and dyspnea after administration (maternal toxicity), as well as prolonged estrus cycle, increased early embryonic deaths and decreased numbers of live fetuses. In addition, low fetal body weights and a reduced number of ossified sacral and caudal vertebrae (ie, retarded fetal development) occurred in rats at 20 times the human dose. Findings in pregnant rabbits consisted of decreased fecal/urine eliminations (the only toxicity noted at 1 mcg/kg/day in dams) as well as decreased food consumption, body weight loss, abortion, increased embryonic and fetal deaths, and decreased numbers of live fetuses. No teratogenic effects of Oprelvekin were observed in rabbits at doses up to 0.6 times the human dose (30 mcg/kg/day).
- Adverse effects in the first generation offspring of rats given Oprelvekin at maternally toxic doses ≥2 times the human dose (≥100 mcg/kg/day) during both gestation and lactation included increased newborn mortality, decreased viability index on day 4 of lactation, and decreased body weights during lactation. In rats given 20 times the human dose (1000 mcg/kg/day) during both gestation and lactation, maternal toxicity and growth retardation of the first generation offspring resulted in an increased rate of fetal death of the second generation offspring.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Oprelvekin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Oprelvekin during labor and delivery.
Nursing Mothers
It is not known if Oprelvekin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Oprelvekin, a decision should be made whether to discontinue nursing or to discontinue Oprelvekin, taking into account the importance of the drug to the mother.
Pediatric Use
A safe and effective dose of Oprelvekin has not been established in children. In a Phase 1, single arm, dose-escalation study, 43 pediatric patients were treated with Oprelvekin at doses ranging from 25 to 125 mcg/kg/day following ICE chemotherapy. All patients required platelet transfusions and the lack of a comparator arm made the study design inadequate to assess efficacy. The projected effective dose (based on comparable AUC observed for the effective dose in healthy adults) in children appears to exceed the maximum tolerated pediatric dose of 50 mcg/kg/day. Papilledema was dose-limiting and occurred in 16% of children.
- The most common adverse events seen in pediatric studies included tachycardia (84%), conjunctival injection (57%), radiographic and echocardiographic evidence of cardiomegaly (21%) and periosteal changes (11%). These events occurred at a higher frequency in children than adults. The incidence of other adverse events was generally similar to those observed using Oprelvekin at a dose of 50 mcg/kg in the randomized studies in adults receiving chemotherapy.
- Studies in animals were predictive of the effect of Oprelvekin on developing bone in children. In growing rodents treated with 100, 300, or 1000 mcg/kg/day for a minimum of 28 days, thickening of femoral and tibial growth plates was noted, which did not completely resolve after a 28-day non-treatment period. In a nonhuman primate toxicology study of Oprelvekin animals treated for two to 13 weeks at doses of 10 to 1000 mcg/kg showed partially reversible joint capsule and tendon fibrosis and periosteal hyperostosis. An asymptomatic, laminated periosteal reaction in the diaphyses of the femur, tibia, and fibula has been observed in one patient during pediatric studies involving multiple courses of Oprelvekin treatment. The relationship of these findings to treatment with Oprelvekin is unclear. No studies have been performed to assess the long-term effects of Oprelvekin on growth and development.
Geriatic Use
- Clinical studies of Oprelvekin did not include sufficient numbers of patients aged 65 and older to determine whether they respond differently from younger subjects. In a controlled study, 141 adult patients with various nonmyeloid malignancies were randomized (2:1) to Oprelvekin 50 mcg/kg/day or placebo administered subcutaneously for 14 days after chemotherapy was completed. Among 106 patients less than 65 years of age, the proportion who did not require platelet transfusions was higher among Oprelvekin-treated patients (36.5% vs. 14.3%). Among 35 patients greater than or equal to 65 years of age, the proportion who did not require platelet transfusions was similar between treatment groups (32% vs. 30%, Oprelvekin and placebo, respectively).
Gender
There is no FDA guidance on the use of Oprelvekin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Oprelvekin with respect to specific racial populations.
Renal Impairment
- Oprelvekin is eliminated primarily by the kidneys. The pharmacokinetics of Oprelvekin were studied in subjects with varying degrees of renal dysfunction. AUC0-∞, Cmax, and absolute bioavailability were significantly increased in subjects with severe renal impairment (creatinine clearance < 30 mL/min). There were no significant changes in the pharmacokinetic parameters in subjects with mild or moderate impairment. A significant decrease in the hemoglobin concentration was noted on Day 2 after a single dose of Oprelvekin in subjects with all degrees of renal impairment. By Day 14, the hemoglobin was decreased only in patients with severe renal impairment. Fluid retention associated with Oprelvekin treatment has not been studied in patients with renal impairment, but fluid balance should be carefully monitored in these patients
Hepatic Impairment
There is no FDA guidance on the use of Oprelvekin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Oprelvekin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Oprelvekin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Oprelvekin Administration in the drug label.
Monitoring
There is limited information regarding Oprelvekin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Oprelvekin and IV administrations.
Overdosage
- Doses of Oprelvekin above 125 mcg/kg have not been administered to humans. While clinical experience is limited, doses of Oprelvekin greater than 50 mcg/kg may be associated with an increased incidence of cardiovascular events in adult patients. If an overdose of Oprelvekin is administered, Oprelvekin should be discontinued, and the patient should be closely observed for signs of toxicity. Reinstitution of Oprelvekin therapy should be based upon individual patient factors (eg, evidence of toxicity, continued need for therapy).
Pharmacology
Oprelvekin
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | L03 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | approx. 19,000 daltons |
| Pharmacokinetic data | |
| Bioavailability | >80% (s.c. application) |
| Metabolism | mainly renal |
| Half life | 6.9 ± 1.7h |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status |
Rx only. Not a controlled substance. |
| Routes | s.c. injection |
Mechanism of Action
- The primary hematopoietic activity of Oprelvekin is stimulation of megakaryocytopoiesis and thrombopoiesis. Oprelvekin has shown potent thrombopoietic activity in animal models of compromised hematopoiesis, including moderately to severely myelosuppressed mice and nonhuman primates. In these models, Oprelvekin improved platelet nadirs and accelerated platelet recoveries compared to controls.
- IL-11 has also been shown to have non-hematopoietic activities in animals including the regulation of intestinal epithelium growth (enhanced healing of gastrointestinal lesions), the inhibition of adipogenesis, the induction of acute phase protein synthesis, inhibition of pro-inflammatory cytokine production by macrophages, and the stimulation of osteoclastogenesis and neurogenesis. Non-hematopoietic pathologic changes observed in animals include fibrosis of tendons and joint capsules, periosteal thickening, papilledema, and embryotoxicity.
- IL-11 is produced by bone marrow stromal cells and is part of the cytokine family that shares the gp130 signal transducer. Primary osteoblasts and mature osteoclasts express mRNAs for both IL-11 receptor (IL-11R alpha) and gp130. Both bone-forming and bone-resorbing cells are potential targets of IL-11. (1)
Structure
There is limited information regarding Oprelvekin Structure in the drug label.
Pharmacodynamics
- In a study in which Oprelvekin was administered to non-myelosuppressed cancer patients, daily subcutaneous dosing for 14 days with Oprelvekin increased the platelet count in a dose-dependent manner. Platelet counts began to increase relative to baseline between five and nine days after the start of dosing with Oprelvekin After cessation of treatment, platelet counts continued to increase for up to seven days then returned toward baseline within 14 days. No change in platelet reactivity as measured by platelet activation in response to ADP, and platelet aggregation in response to ADP, epinephrine, collagen, ristocetin and arachidonic acid has been observed in association with Oprelvekin treatment.
- In a randomized, double-blind, placebo-controlled study in normal volunteers, subjects receiving Oprelvekin had a mean increase in plasma volume of >20%, and all subjects receiving Oprelvekin had at least a 10% increase in plasma volume. Red blood cell volume decreased similarly (due to repeated phlebotomy) in the Oprelvekin and placebo groups. As a result, whole blood volume increased approximately 10% and hemoglobin concentration decreased approximately 10% in subjects receiving Oprelvekin compared with subjects receiving placebo. Mean 24 hour sodium excretion decreased, and potassium excretion did not increase, in subjects receiving Oprelvekin compared with subjects receiving placebo.
Pharmacokinetics
- The pharmacokinetics of Oprelvekin have been evaluated in studies of healthy, adult subjects and cancer patients receiving chemotherapy. In a study in which a single 50 mcg/kg subcutaneous dose was administered to eighteen healthy men, the peak serum concentration (Cmax) of 17.4 ± 5.4 ng/mL (mean ± S.D.) was reached at 3.2 ± 2.4 hrs (Tmax) following dosing. The terminal half-life was 6.9 ± 1.7 hrs. In a second study in which single 75 mcg/kg subcutaneous and intravenous doses were administered to twenty-four healthy subjects, the pharmacokinetic profiles were similar between men and women. The absolute bioavailability of Oprelvekin was >80%. In a study in which multiple, subcutaneous doses of both 25 and 50 mcg/kg were administered to cancer patients receiving chemotherapy, Oprelvekin did not accumulate and clearance of Oprelvekin was not impaired following multiple doses.
- Oprelvekin was administered at doses ranging from 25 to 125 mcg/kg/day to 43 pediatric patients (ages 8 months to 18 years) and 1 adult patient receiving ICE (ifosfamide, carboplatin, etoposide) chemotherapy. Analysis of data from 40 pediatric patients showed that Cmax, Tmax, and terminal half-life were comparable to that in adults. The mean area under the concentration-time curve (AUC) for pediatric patients (8 months to 18 years), receiving 50 mcg/kg was approximately half that achieved in healthy adults receiving 50 mcg/kg. Available data suggest that clearance of Oprelvekin decreases with increasing age in children.
- Oprelvekin was administered as a single 50 mcg/kg dose subcutaneously to 48 healthy male and female adults aged 20 to 79 years; 18 subjects were aged 65 or older. The pharmacokinetic profile of Oprelvekin was similar between those 65 years of age or older and those younger than 65 years.
- In preclinical trials in rats, radiolabeled Oprelvekin was rapidly cleared from the serum and distributed to highly perfused organs. The kidney was the primary route of elimination. The amount of intact Oprelvekin in urine was low, indicating that the molecule was metabolized before excretion. In a clinical study, a single dose of Oprelvekin was administered to subjects with severely impaired renal function (creatinine clearance <30 mL/min). The mean ± S.D. values for Cmax and AUC were 30.8 ± 8.6 ng/mL and 373 ± 106 ng*hr/mL, respectively. When compared with control subjects in this study with normal renal function, the mean Cmax was 2.2 fold higher and the mean AUC was 2.6 fold (95% confidence interval, 1.7%-3.8%) higher in the subjects with severe renal impairment. In the subjects with severe renal impairment, clearance was approximately 40% of the value seen in subjects with normal renal function. The average terminal half-life was similar in subjects with severe renal impairment and those with normal renal function.
- A second clinical study of 24 subjects with varying degrees of renal function was also performed and confirmed the results observed in the first study. Single 50 mcg/kg subcutaneous and intravenous doses were administered in a randomized fashion. As the degree of renal impairment increased, the Oprelvekin AUC increased, although half-life remained unchanged. In the six patients with severe impairment, the mean ± S.D. Cmax and AUC were 23.6 ± 6.7 ng/mL and 373 ± 55.2 ng*hr/mL, respectively, compared with 13.1 ± 3.8 ng/mL and 195 ± 49.3 ng*hr/mL, respectively, in the six subjects with normal renal function. A comparable increase in exposure was observed after intravenous administration of Oprelvekin.
- The pharmacokinetic studies suggest that overall exposure to oprelvekin increases as renal function decreases, indicating that a 50% dose reduction of Oprelvekin is warranted for patients with severe renal impairment. No dosage reduction is required for smaller changes in renal function.
Nonclinical Toxicology
There is limited information regarding Oprelvekin Nonclinical Toxicology in the drug label.
Clinical Studies
Two randomized, double-blind, placebo-controlled trials in adults studied Oprelvekin for the prevention of severe thrombocytopenia following single or repeated sequential cycles of various myelosuppressive chemotherapy regimens.
Study in Patients with Prior Chemotherapy-Induced Thrombocytopenia
- One study evaluated the effectiveness of Oprelvekin in eliminating the need for platelet transfusions in patients who had recovered from an episode of severe chemotherapy-induced thrombocytopenia (defined as a platelet count ≤20,000/μL), and were to receive one additional cycle of the same chemotherapy without dose reduction. Patients had various underlying non-myeloid malignancies, and were undergoing dose-intensive chemotherapy with a variety of regimens. Patients were randomized to receive Oprelvekin at a dose of 25 mcg/kg or 50 mcg/kg, or placebo. The primary endpoint was whether the patient required one or more platelet transfusions in the subsequent chemotherapy cycle. Ninety-three patients were randomized. Five patients withdrew from the study prior to receiving the study drug. As a result, eighty-eight patients were included in a modified intent-to-treat analysis. The results for the Oprelvekin 50 mcg/kg and placebo groups are summarized in TABLE 1. The placebo group includes one patient who underwent chemotherapy dose reduction and who avoided platelet transfusions.
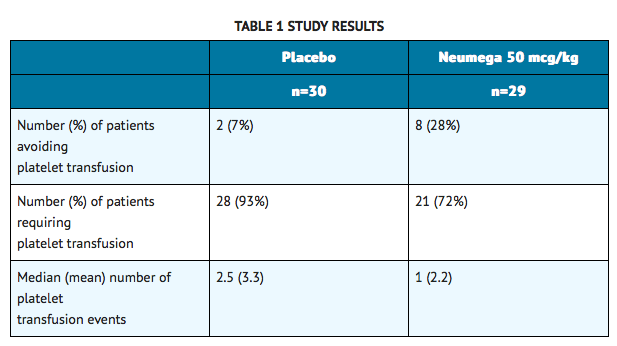
- In the primary efficacy analysis, more patients avoided platelet transfusion in the Oprelvekin 50 mcg/kg arm than in the placebo arm (p = 0.04, Fisher's Exact test, 2-tailed). The difference in the proportion of patients avoiding platelet transfusions in the Oprelvekin 50 mcg/kg and placebo groups was 21% (95% confidence interval, 2%-40%). The results observed in patients receiving 25 mcg/kg of Oprelvekin were intermediate between those of the placebo and the 50 mcg/kg groups.
Study in Patients Receiving Dose-Intensive Chemotherapy
- A second study evaluated the effectiveness of Oprelvekin in eliminating platelet transfusions over two dose-intensive chemotherapy cycles in breast cancer patients who had not previously experienced severe chemotherapy-induced thrombocytopenia. All patients received the same chemotherapy regimen (cyclophosphamide 3,200 mg/m2 and doxorubicin 75 mg/m2). All patients received concomitant filgrastim (G-CSF) in all cycles. The patients were stratified by whether or not they had received prior chemotherapy, and randomized to receive Oprelvekin 50 mcg/kg or placebo. The primary endpoint was whether or not a patient required one or more platelet transfusions in the two study cycles. Seventy-seven patients were randomized. Thirteen patients failed to complete both study cycles—eight of these had insufficient data to be evaluated for the primary endpoint. The results of this trial are summarized in TABLE 2.
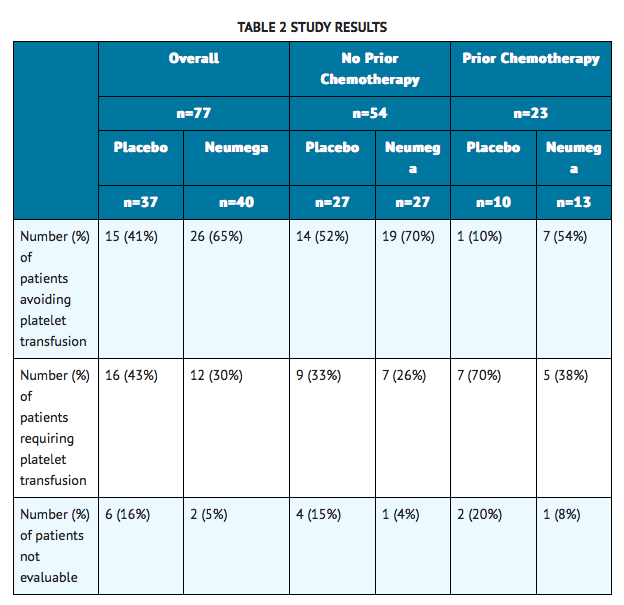
- This study showed a trend in favor of Oprelvekin, particularly in the subgroup of patients with prior chemotherapy. Open-label treatment with Oprelvekin has been continued for up to four consecutive chemotherapy cycles without evidence of any adverse effect on the rate of neutrophil recovery or red blood cell transfusion requirements. Some patients continued to maintain platelet nadirs >20,000/μL for at least four sequential cycles of chemotherapy without the need for transfusions, chemotherapy dose reduction, or changes in treatment schedules.
- Platelet activation studies done on a limited number of patients showed no evidence of abnormal spontaneous platelet activation, or an abnormal response to ADP. In an unblinded, retrospective analysis of the two placebo-controlled studies, 19 of 69 patients (28%) receiving Oprelvekin 50 mcg/kg and 34 of 67 patients (51%) receiving placebo reported at least one hemorrhagic adverse event which involved bleeding.
How Supplied
There is limited information regarding Oprelvekin How Supplied in the drug label.
Storage
There is limited information regarding Oprelvekin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Oprelvekin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Oprelvekin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Oprelvekin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Oprelvekin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Oprelvekin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.