Olsalazine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Olsalazine is a Salicylate that is FDA approved for the treatment of ulcerative colitis. Common adverse reactions include Abdominal pain, Diarrhea, Indigestion, Nausea, Headache, Blurred vision.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Olsalazine is indicated for the maintenance of remission of ulcerative colitis in patients who are intolerant of sulfasalazine.
Dosage
- The usual dosage in adults for maintenance of remission is 1.0 g/day in two divided doses.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Olsalazine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Olsalazine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Olsalazine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Olsalazine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Olsalazine in pediatric patients.
Contraindications
- Hypersensitivity to olsalazine, other salicylates, or any of the excipients.
Warnings
Precautions
General
- Overall, approximately 17% of subjects receiving olsalazine in clinical studies reported diarrhea sometime during therapy. This diarrhea resulted in withdrawal of treatment in 6% of patients. This diarrhea appears to be dose related, although it may be difficult to distinguish from the underlying symptoms of the disease.
- Exacerbation of the symptoms of colitis thought to have been caused by mesalamine or sulfasalazine has been noted.
Information for Patients
- Patients should be instructed to take olsalazine with food. The drug should be taken in evenly divided doses. Patients should be informed that about 17% of subjects receiving olsalazine during clinical studies reported diarrhea sometime during therapy. If diarrhea occurs, patients should contact their physician.
Adverse Reactions
Clinical Trials Experience
- Olsalazine has been evaluated in ulcerative colitis patients in remission, as well as those with acute disease. Both sulfasalazine-tolerant and intolerant patients have been studied in controlled clinical trials. Overall, 10.4% of patients discontinued olsalazine because of an adverse experience compared with 6.7% of placebo patients. The most commonly reported adverse reactions leading to treatment withdrawal were diarrhea or loose stools (olsalazine 5.9%; placebo 4.8%), abdominal pain, and rash or itching (slightly more than 1% of patients receiving olsalazine). Other adverse reactions to olsalazine leading to withdrawal occurred in fewer than 1% of patients (TABLE 1).
- For those controlled studies, the comparative incidences of adverse reactions reported in 1% or more patients treated with olsalazine or placebo are provided in Table 2.
- Over 2,500 patients have been treated with olsalazine in various controlled and uncontrolled clinical studies. In these as well as in post-marketing experience, olsalazine was administered mainly to patients intolerant to sulfasalazine. There have been rare reports of the following adverse effects in patients receiving olsalazine. These were often difficult to distinguish from possible symptoms of the underlying disease or from the effects of prior and/or concomitant therapy. A causal relationship to the drug has not been demonstrated for some of these reactions.
Blood and Lymphatic System Disorders
- Anemia, Eosinophilia, Hemolytic anemia, Interstitial pulmonary disease, Leukopenia, Lymphopenia, Neutropenia, Reticulocytosis, Thrombocytopenia
Cardiac Disorders
- Chest pains, Heart block second degree, Myocarditis, Palpitations, Pericarditis, Peripheral edema, Shortness of breath, Tachycardia
- A patient who developed thyroid disease 9 days after starting DIPENTUM was given propranolol and radioactive iodine and subsequently developed shortness of breath and nausea. The patient died 5 days later with signs and symptoms of acute diffuse myocarditis.
Ear and Labyrinth Disorders
Eye Disorders
Gastrointestinal Disorders
- Abdominal pain (upper), Diarrhea with dehydration, Dry mouth, Epigastric discomfort, Flare in symptoms, Flatulence, Increased blood in stool, Pancreatitis, Rectal bleeding, Rectal discomfort
- In a double-blind, placebo-controlled study, increased frequency and severity of diarrhea were reported in patients randomized to olsalazine 500 mg B.I.D. with concomitant pelvic radiation.
- Rare cases of granulomatous hepatitis and nonspecific, reactive hepatitis have been reported in patients receiving olsalazine. Additionally, a patient developed mild cholestatic hepatitis during treatment with sulfasalazine and experienced the same symptoms two weeks later after the treatment was changed to olsalazine. Withdrawal of olsalazine led to complete recovery in these cases.
General Disorders and Administration Site Conditions
- Fever chills, Hot flashes, Irritability, Rigors
Immune System Disorders
Laboratory
Musculoskeletal and Connective Tissue Disorders
Nervous System Disorders
Psychiatric Disorders
Renal and Urinary Disorders
- Dysuria, Hematuria, Interstitial nephritis, Nephrotic syndrome, Proteinuria, Urinary frequency
Reproductive System and Breast Disorders
Skin and Subcutaneous Tissue Disorders
Vascular Disorders
Postmarketing Experience
- The following events have been identified during post-approval use of products that contain (or are metabolized to) mesalamine in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of seriousness, frequency of reporting, or potential causal connection to mesalamine:
Blood and Lymphatic System Disorders
General Disorders and Administration Site Conditions
Hepatobiliary Disorders
- Hepatic enzyme increased, Hepatitis, Increased bilirubin
- Reports of hepatotoxicity, including elevated liver function tests (SGOT/AST, SGPT/ALT, GGT, LDH, alkaline phosphatase, bilirubin), jaundice, cholestatic jaundice, cirrhosis, and possible hepatocellular damage including liver necrosis and liver failure. Some of these cases were fatal. One case of Kawasaki-like syndrome, which included hepatic function changes, was also reported.
Musculoskeletal and Connective Tissue Disorders
Respiratory, Thoracic and Mediastinal Disorders
Skin and Subcutaneous Tissue Disorders
Nervous System Disorders
Renal and Urinary Disorders
Drug Interactions
- The co-administration of salicylates and low molecular weight heparins or heparinoids may result in an increased risk of bleeding (i.e., hematomas) following neuraxial anesthesia. Salicylates should be discontinued prior to the initiation of a low molecular weight heparin or heparinoid. If this is not possible, it is recommended to monitor patients closely for bleeding.
- Increased prothrombin time in patients taking concomitant warfarin has been reported.
- The co-administration of olsalazine and 6-mercaptopurine or thioguanine may result in an increased risk of myelosuppression. If co-administered with 6-mercaptopurine, it is recommended to use the lowest possible doses of each drug and to monitor the patient, especially for leukopenia. In case of co-administration with thioguanine, careful monitoring of blood counts is recommended.
- It is recommended not to give salicylates for six weeks after the varicella vaccine to avoid a possible increased risk of developing Reye’s syndrome.
Drug/Laboratory Test Interactions
- None known.
Use in Specific Populations
Pregnancy
- Olsalazine has been shown to produce fetal developmental toxicity as indicated by reduced fetal weights, retarded ossifications, and immaturity of the fetal visceral organs when given during organogenesis to pregnant rats in doses 5 to 20 times the human dose (100 to 400 mg/kg).
- There are no adequate and well-controlled studies in pregnant women. Olsalazine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Olsalazine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Olsalazine during labor and delivery.
Nursing Mothers
- Small amounts of the active metabolite of olsalazine (5-ASA) may pass into breast milk. Harmful infant effects (diarrhea) have been reported when 5-ASA was used during breastfeeding. Unless the benefit of the treatment outweighs the risks, olsalazine should not be taken by breast-feeding women, or patients should be advised to discontinue breastfeeding if using olsalazine.
- Oral administration of olsalazine to lactating rats in doses 5 to 20 times the human dose produced growth retardation in their pups.
Pediatric Use
- Safety and effectiveness in a pediatric population have not been established.
Geriatic Use
- Clinical studies of DIPENTUM did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, elderly patients should be treated with caution due to the greater frequency of decreased hepatic, renal, or cardiac function, co-existence of other disease, as well as concomitant drug therapy.
Gender
There is no FDA guidance on the use of Olsalazine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Olsalazine with respect to specific racial populations.
Renal Impairment
- Patients with impaired renal function should be monitored.
- Although renal abnormalities were not reported in clinical trials with olsalazine, there have been rare reports from post-marketing experience. Therefore, the possibility of renal tubular damage due to absorbed mesalamine or its n-acetylated metabolite, as noted in the ANIMAL TOXICOLOGY section must be kept in mind, particularly for patients with pre-existing renal disease. In these patients, monitoring with urinalysis, BUN, and creatinine determinations is advised.
Hepatic Impairment
Patients with impaired hepatic function should be monitored
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Olsalazine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Olsalazine in patients who are immunocompromised.
Severe Allergies and/or Asthma
- Patients with severe allergies or asthma should be monitored for signs of worsening symptoms.
Administration and Monitoring
Administration
- Oral
Monitoring
- If co-administered with 6-mercaptopurine, it is recommended to use the lowest possible doses of each drug and to monitor the patient, especially for leukopenia. In case of co-administration with thioguanine, careful monitoring of blood counts is recommended.
- Patients with severe allergies or asthma should be monitored for signs of worsening symptoms
- Patients with impaired renal function should be monitored.
- For patients with pre-existing renal disease monitoring with urinalysis, BUN, and creatinine determinations is advised.
- Patients with impaired hepatic function should be monitored
IV Compatibility
There is limited information regarding IV Compatibility of Olsalazine in the drug label.
Overdosage
- No overdosage has been reported in humans. The knowledge of overdosage is limited. Possible overdose symptoms include nausea, vomiting and diarrhea. It is recommended to check hematology, acid-base, electrolyte, liver and kidney status, and to provide supportive treatment. There is no specific antidote to DIPENTUM.
- Maximum single oral doses of 5g/kg in mice and rats and 2 g/kg in dogs were not lethal. Symptoms of acute toxicity were decreased motor activity and diarrhea in all species tested. In addition, vomiting was reported in dogs.
DRUG ABUSE AND DEPENDENCY
Abuse
- None reported.
Dependence
- Drug dependence has not been reported with chronic administration of olsalazine.
Pharmacology
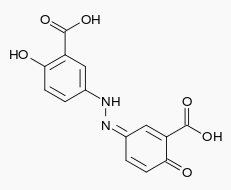
| |
Olsalazine
| |
| Systematic (IUPAC) name | |
| 5-[(2Z)-2-(3-carboxy-4-oxocyclohexa-2,5-dien-1-ylidene)hydrazino]-2-hydroxybenzoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | A07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 302.239g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 99% |
| Metabolism | ? |
| Half life | 0.9 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- The mechanism of action of mesalamine (and sulfasalazine) is unknown, but appears to be topical rather than systemic. Mucosal production of arachidonic acid (AA) metabolites, both through the cyclooxygenase pathways (i.e., prostanoids) and through the lipoxygenase pathways (i.e., leukotrienes [LTs] and hydroxyeicosatetraenoic acids [HETEs]) is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin (PG) production in the colon.
Structure
- The active ingredient in DIPENTUM Capsules (olsalazine sodium) is the sodium salt of a salicylate, disodium 3,3'-azobis (6-hydroxybenzoate) a compound that is effectively bioconverted to 5-aminosalicylic acid (5-ASA), which has anti-inflammatory activity in ulcerative colitis. Its empirical formula is C14H8N2Na2O6 with a molecular weight of 346.21.
- The structural formula is:
- Olsalazine sodium is a yellow crystalline powder, which melts with decomposition at 240°C. It is the sodium salt of a weak acid, soluble in water and DMSO, and practically insoluble in ethanol, chloroform, and ether. Olsalazine sodium has acceptable stability under acidic or basic conditions.
- DIPENTUM is supplied in hard gelatin capsules for oral administration. The inert ingredient in each 250 mg capsule of olsalazine sodium is magnesium stearate. The capsule shell contains the following inactive ingredients: black iron oxide, caramel, gelatin, and titanium dioxide.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Olsalazine in the drug label.
Pharmacokinetics
- After oral administration, olsalazine has limited systemic bioavailability. Based on oral and intravenous dosing studies, approximately 2.4% of a single 1.0 g oral dose is absorbed. Less than 1% of olsalazine is recovered in the urine. The remaining 98 to 99% of an oral dose will reach the colon, where each molecule is rapidly converted into two molecules of 5-aminosalicylic acid (5-ASA) by colonic bacteria and the low prevailing redox potential found in this environment. The liberated 5-ASA is absorbed slowly resulting in very high local concentrations in the colon.
- The conversion of olsalazine to mesalamine (5-ASA) in the colon is similar to that of sulfasalazine, which is converted into sulfapyridine and mesalamine. It is thought that the mesalamine component is therapeutically active in ulcerative colitis (A.K. Azad-Kahn et al, LANCET, 2: 892-895, 1977). The usual dose of sulfasalazine for maintenance of remission in patients with ulcerative colitis is 2 grams daily, which would provide approximately 0.8 grams of mesalamine to the colon. More than 0.9 grams of mesalamine would usually be made available in the colon from 1 gram of olsalazine.
- The pharmacokinetics of olsalazine are similar in both healthy volunteers and in patients with ulcerative colitis. Maximum serum concentrations of olsalazine appear after approximately 1 hour and, even after a 1.0 g single dose, are low (e.g., 1.6 to 6.2 µmol/L). Olsalazine has a very short serum half-life, approximately 0.9 hours. Olsalazine is more than 99% bound to plasma proteins. It does not interfere with protein binding of warfarin.The urinary recovery of olsalazine is below 1%. Total recovery of oral 14C-labeled olsalazine in animals and humans ranges from 90 to 97%. Approximately 0.1% of an oral dose of olsalazine is metabolized in the liver to olsalazine-O-sulfate (olsalazine-S). Olsalazine-S, in contrast to olsalazine has a half-life of 7 days. Olsalazine-S accumulates to steady state within 2 to 3 weeks.
- Patients on daily doses of 1.0 g olsalazine for 2 to 4 years show a stable plasma concentration of olsalazine-S (3.3 to 12.4 µmol/L). Olsalazine-S is more than 99% bound to plasma proteins. Its long half-life is mainly due to slow dissociation from the protein binding site. Less than 1% of both olsalazine and olsalazine-S appears undissociated in plasma.
5-aminosalicylic acid (5-ASA)
- Serum concentrations of 5-ASA are detected after 4 to 8 hours. The peak levels of 5-ASA after an oral dose of 1.0 g olsalazine are low (i.e., 0 to 4.3 µmol/L). Of the total 5-ASA found in the urine, more than 90% is in the form of N-acetyl-5-ASA (Ac-5-ASA). Only small amounts of 5-ASA are detected.
- N-acetyl-5-ASA (Ac-5-ASA), the major metabolite of 5-ASA found in plasma and urine, is acetylated (deactivated) in at least two sites, the colonic epithelium and the liver. Ac-5-ASA is found in the serum, with peak values of 1.7 to 8.7 µmol/L after a single 1.0 g dose. Approximately 20% of the total 5-ASA is recovered in the urine, where it is found almost exclusively as Ac-5-ASA. The remaining 5-ASA is partially acetylated and is excreted in the feces. From fecal dialysis, the concentration of 5-ASA in the colon following olsalazine has been calculated to be 18 to 49 mmol/L. No accumulation of 5-ASA or Ac-5-ASA in plasma has been detected. 5-ASA and Ac-5-ASA are 74 and 81%, respectively, bound to plasma proteins.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a two year oral rat carcinogenicity study, olsalazine was tested in male and female Wistar rats at daily doses of 200, 400, and 800 mg/kg/day (approximately 10 to 40 times the human maintenance dose, based on a patient weight of 50 kg and a human dose of 1 g). Urinary bladder transitional cell carcinomas were found in three male rats (6%, p=0.022, exact trend test) receiving 40 times the human dose and were not found in untreated male controls. In the same study, urinary bladder transitional cell carcinoma and papilloma occurred in 2 untreated control female rats (2%). No such tumors were found in any of the female rats treated at doses up to 40 times the human dose.
- In an eighteen month oral mouse carcinogenicity study, olsalazine was tested in male and female CD-1 mice at daily doses of 500, 1000, and 2000 mg/kg/day (approximately 25 to 100 times the human maintenance dose). Liver hemangiosarcomata were found in two male mice (4%) receiving olsalazine at 100 times the human dose, while no such tumor occurred in the other treated male mice groups or any of the treated female mice. The observed incidence of this tumor is within the 4% incidence in historical controls.
- Olsalazine was not mutagenic in in vitro Ames tests, mouse lymphoma cell mutation assays, human lymphocyte chromosomal aberration tests, or the in vivo rat bone marrow cell chromosomal aberration test.
- Olsalazine in a dose range of 100 to 400 mg/kg/day (approximately 5 to 20 times the human maintenance dose) did not influence the fertility of male or female rats. The oligospermia and infertility in men associated with sulfasalazine have not been reported with olsalazine.
ANIMAL TOXICOLOGY
- Preclinical subacute and chronic toxicity studies in rats have shown the kidney to be the major target organ of olsalazine toxicity. At an oral daily dose of 400 mg/kg or higher, olsalazine treatment produced nephritis and tubular necrosis in a 4-week study; interstitial nephritis and tubular calcinosis in a 6-month study, and renal fibrosis, mineralization, and transitional cell hyperplasia in a 1-year study.
Clinical Studies
- Two controlled studies have demonstrated the efficacy of olsalazine as maintenance therapy in patients with ulcerative colitis. In the first, ulcerative colitis patients in remission were randomized to olsalazine 500 mg B.I.D. or placebo, and relapse rates for a six month period of time were compared. For the 52 patients randomized to olsalazine, 12 relapses occurred, while for the 49 placebo patients, 22 relapses occurred. This difference in relapse rates was significant (p<0.02).
- In the second study, 164 ulcerative colitis patients in remission were randomized to olsalazine 500 mg B.I.D. or sulfasalazine 1 gram B.I.D., and relapse rates were compared after six months. The relapse rate for olsalazine was 19.5% while that for sulfasalazine was 12.2%, a non-significant difference.
How Supplied
- Beige colored capsules, containing 250 mg olsalazine sodium imprinted with “DIPENTUM® 250 mg” on the capsule shell, available as:
- Bottles of 100’s NDC 53014-726-71
- Bottles of 300’s NDC 53014-726-82
Storage
- Store at 20-25°C (77°F). Excursions permitted to 15° to 30°C (59° to 86°F)
Images
Drug Images
{{#ask: Page Name::Olsalazine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Ingredients and Appearance
{{#ask: Label Page::Olsalazine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be instructed to take olsalazine with food. The drug should be taken in evenly divided doses. Patients should be informed that about 17% of subjects receiving olsalazine during clinical studies reported diarrhea sometime during therapy. If diarrhea occurs, patients should contact their physician.
Precautions with Alcohol
- Alcohol-Olsalazine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Dipentum®[1]
Look-Alike Drug Names
There is limited information regarding Olsalazine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.



