Nucleoside
| Nitrogenous base | Nucleoside | Deoxynucleoside |
|---|---|---|
| br/>Adenine |
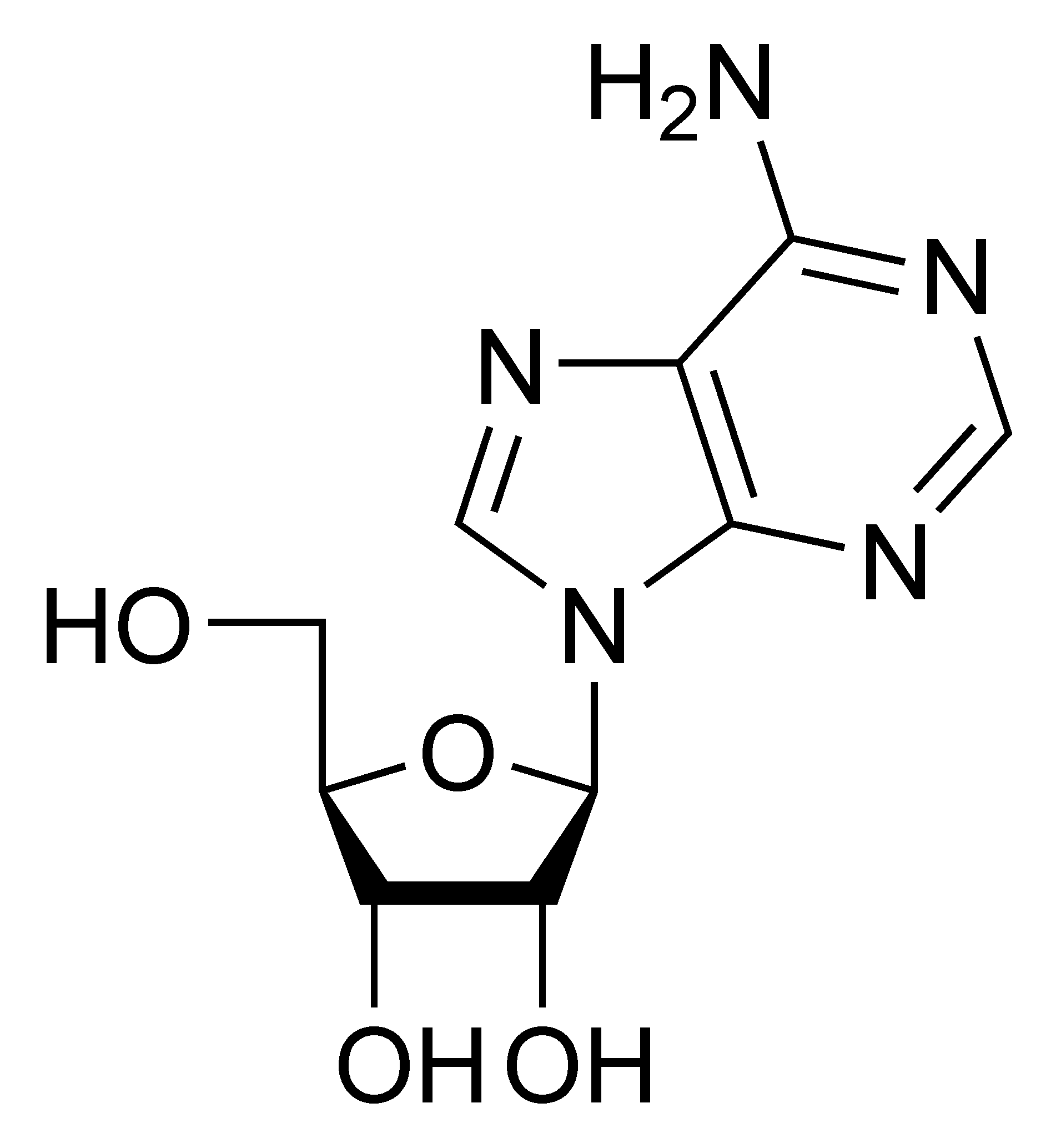 Adenosine A |
Deoxyadenosine dA |
Guanine |
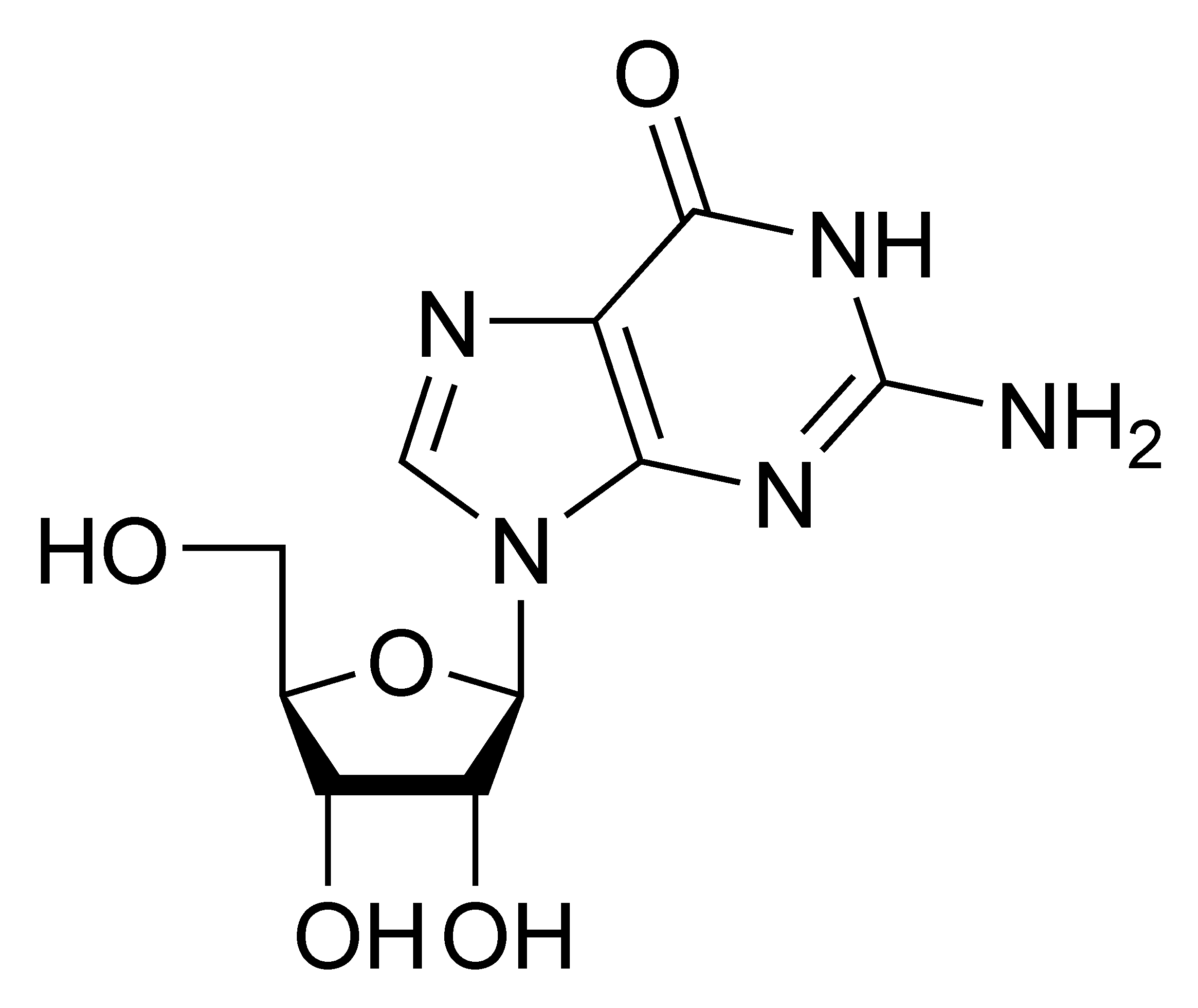 Guanosine G |
Deoxyguanosine dG |
 Thymine |
5-Methyluridine m5U |
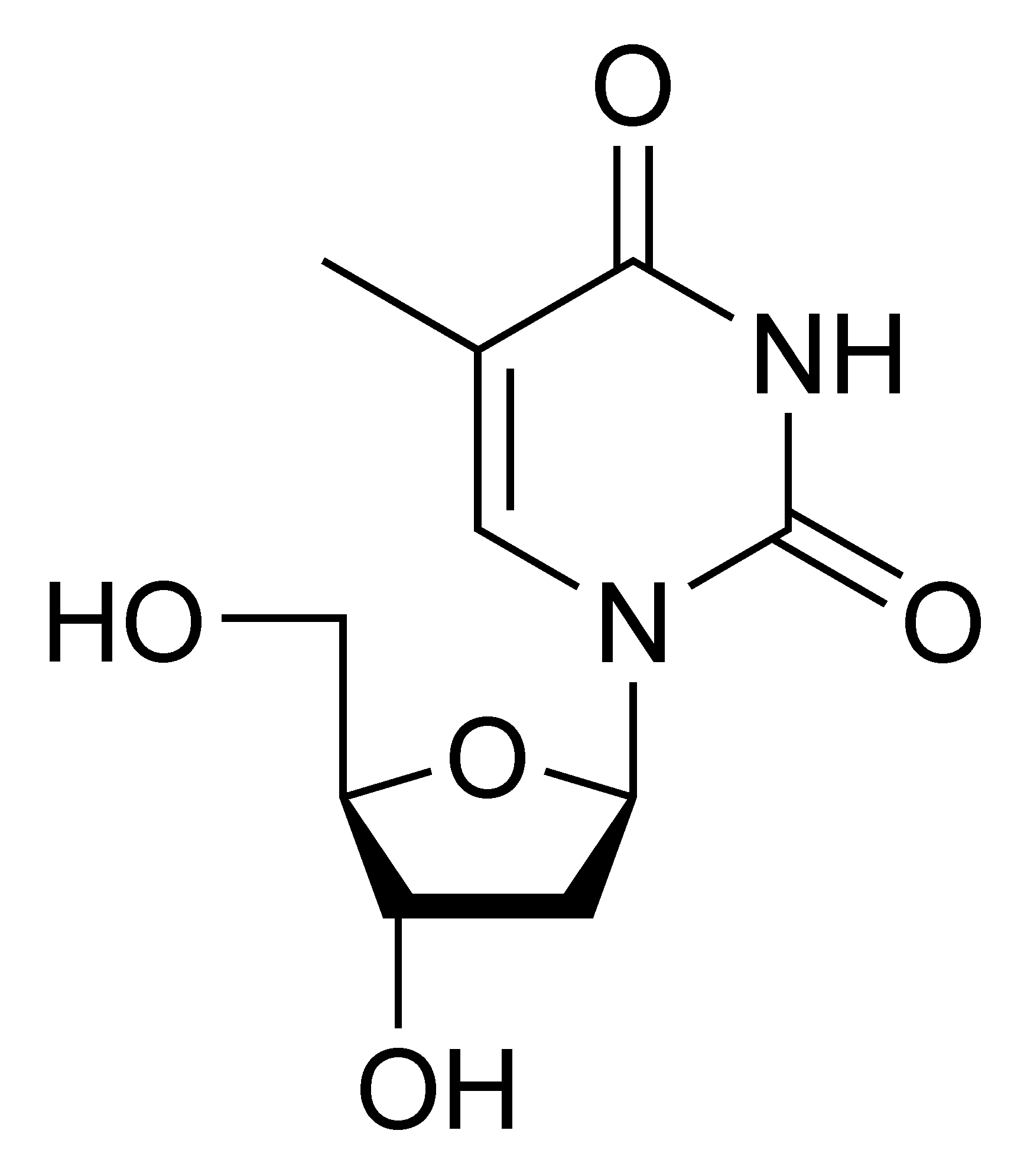 Deoxythymidine dT |
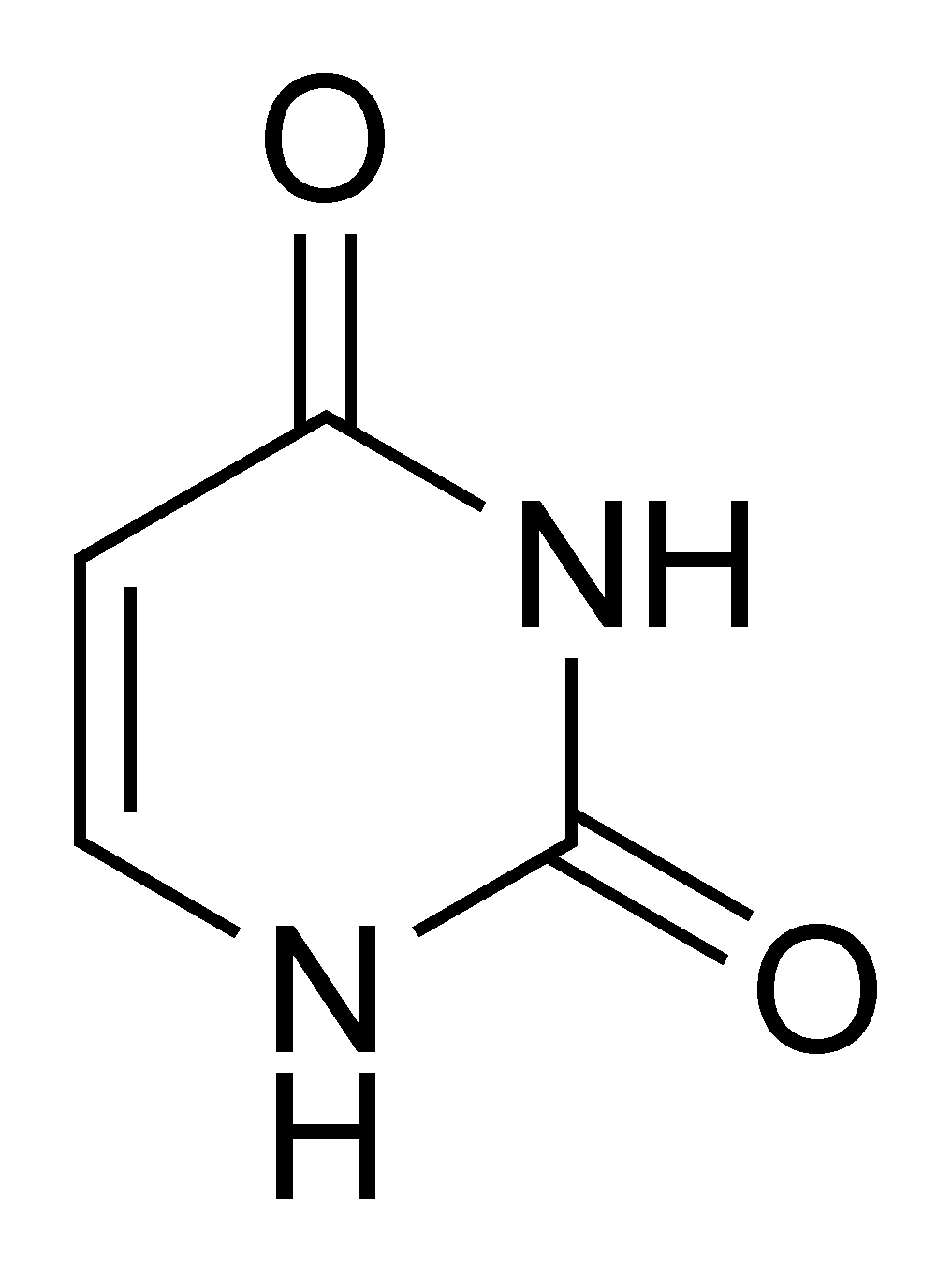 Uracil |
Uridine U |
Deoxyuridine dU |
 Cytosine |
Cytidine C |
Deoxycytidine dC |
|
WikiDoc Resources for Nucleoside |
|
Articles |
|---|
|
Most recent articles on Nucleoside |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Nucleoside at Clinical Trials.gov Clinical Trials on Nucleoside at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Nucleoside
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Nucleoside Discussion groups on Nucleoside Patient Handouts on Nucleoside Directions to Hospitals Treating Nucleoside Risk calculators and risk factors for Nucleoside
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Nucleoside |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Nucleosides are glycosylamines made by attaching a nucleobase (often referred to simply as base) to a ribose or deoxyribose ring. Examples of these include cytidine, uridine, adenosine, guanosine, thymidine and inosine. In short, a nucleoside is a base linked to sugar.
Nucleosides can be phosphorylated by specific kinases in the cell, producing nucleotides, which are the molecular building blocks of DNA and RNA.
Nucleosides are produced as the second step in nucleic acid digestion, whereby nucleotidases break down nucleotides (such as the thymine nucleotide) into nucleosides (such as thymidine) and phosphate. The nucleosides, in turn, are subsequently broken down
- in the lumen of the digestive system by nucleosidases into nitrogenous bases and ribose (or deoxyribose), and
- inside the cell by nucleoside phosphorylases into nitrogenous bases, and ribose-1-phosphate (or deoxyribose-1-phosphate).
Nucleosides can be produced by combining nucleobases with deoxyribose rings as well.
Nucleosides differ from nucleotides by having a hydroxyl group attached to carbon number 5 (the one that isn't in the ring) of the ribose, rather than one or more phosphate groups.
In medicine several nucleoside analogues are used as antiviral or anticancer agents. The viral polymerase incorporates these compounds with non-canon bases. These compounds are activated in the cells by being converted into nucleotides, they are administered as nuclosides since charged nucleotides cannot easily cross cell membranes.
In molecular biology several analogues of the sugar back bone exist. Due to the low stability of RNA, which is prone to hydrolysis, several more stable alternative nucleoside/nucleotide analogues are used which correctly bind to RNA. This is achieved by using a different backbone sugar. These analogues includ LNA, morpholino, PNA.
In sequencing dideoxynucleotides are used. These nucleotides posses a non-canon sugar, dideoxyribose which lacks 3' hydroxyl group (which accepts the phosphate) and therefore cannot bond with the next base, terminating the chain as DNA polymerases mistake it for a regular deoxyribonucleotide.
See also
Template:Nucleic acids
Template:SIB
ca:Nucleòsid cs:Nukleosid da:Nukleosid de:Nukleoside el:Νουκλεοζίτης gl:Nucleósido id:Nukleosida it:Nucleoside ka:ნუკლეოზიდი lt:Nukleozidas hu:Nukleozid nl:Nucleoside fi:Nukleosidi sv:Nukleosid