Nitroglycerin (Intra-anal ointment)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nitroglycerin (Intra-anal ointment) is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the treatment of moderate to severe pain associated with chronic anal fissure. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- RECTIV™ (nitroglycerin) Ointment 0.4% is indicated for the treatment of moderate to severe pain associated with chronic anal fissure.
Dosage
- Apply 1 inch of ointment (375 mg of ointment equivalent to 1.5 mg of nitroglycerin) intra-anally every 12 hours for up to 3 weeks. A finger covering, such as plastic-wrap, disposable surgical glove or a finger cot, should be placed on the finger to apply the ointment. To obtain a 1.5 mg dose of nitroglycerin, the covered finger is laid alongside the 1 inch dosing line on the carton.
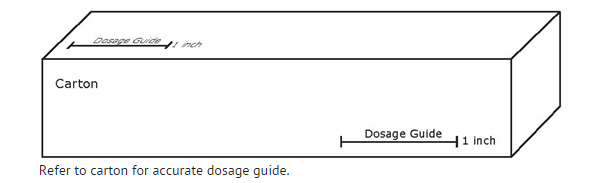
- The tube is gently squeezed until a line of ointment the length of the measuring line is expressed onto the covered finger. The ointment is gently inserted into the anal canal using the covered finger no further than to the first finger joint and the ointment is applied around the side of the anal canal. If this cannot be achieved due to pain, application of the ointment should be made directly to the outside of the anus. Treatment may be continued for up to three weeks.
- RECTIV ointment is not for oral, ophthalmic, or intravaginal use. Hands should be washed after application of the ointment.
DOSAGE FORMS AND STRENGTHS
- Ointment, 0.4% w/w (4 mg/1 g) in 30 g tubes.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitroglycerin (Intra-anal ointment) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitroglycerin (Intra-anal ointment) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Nitroglycerin (Intra-anal ointment) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitroglycerin (Intra-anal ointment) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitroglycerin (Intra-anal ointment) in pediatric patients.
Contraindications
PDE5 inhibitor use
- Administration of RECTIV is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), such as sildenafil, vardenafil, and tadalafil, as these are shown to potentiate the hypotensive effects of organic nitrates.
Severe anemia
RECTIV is contraindicated in patients with severe anemia.
Increased intracranial pressure
- RECTIV is contraindicated in patients with increased intracranial pressure.
Hypersensitivity
- RECTIV is contraindicated in patients who have shown hypersensitivity to it or to other nitrates or nitrites. Skin reactions consistent with hypersensitivity have been observed with organic nitrates.
Warnings
Cardiovascular disorders
- Venous and arterial dilatation as a consequence of nitroglycerin treatment including RECTIV, can decrease venous blood returning to the heart and reduce arterial vascular resistance and systolic pressure. Exercise caution when treating patients with any of the following conditions: blood volume depletion, existing hypotension, cardiomyopathies, congestive heart failure, acute myocardial infarction, or poor cardiac function for other reasons. If patients with any of these conditions are treated with RECTIV, monitor cardiovascular status and clinical condition. The adverse reactions of RECTIV are likely to be more pronounced in the elderly.
Headache
- RECTIV produces dose-related headaches, which may be severe. Tolerance to headaches occurs.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The most common adverse reaction of RECTIV (nitroglycerin) Ointment 0.4% applied to the anal canal is headache.
- Headache may be recurrent following each dose. Headaches are typically of short duration and can be treated with an analgesic, e.g. acetaminophen, and are reversible upon discontinuation of treatment.
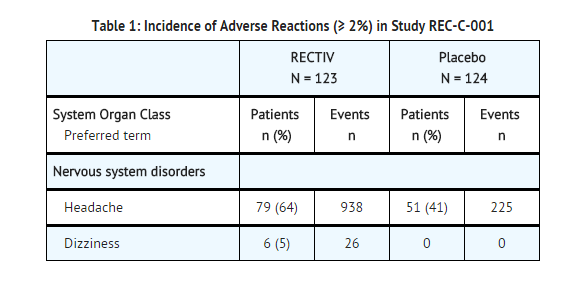
- In Study REC-C-001, a double-blind, placebo-controlled trial in patients with a painful chronic anal fissure, the most frequent (≥ 2%) adverse reactions reported were as follows (Table 1):
Hypotension
Transient episodes of light-headedness, occasionally related to blood pressure changes, also may occur. Hypotension (including orthostatic hypotension) occurs infrequently, but in some patients may be severe enough to warrant discontinuation of therapy.
Allergic Reactions
- Flushing, allergic reactions and application site reactions (including drug rash and exfoliative dermatitis) have been reported rarely.
Methemoglobinemia
- In rare cases, therapeutic doses of organic nitrates have caused methemoglobinemia
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nitroglycerin (Intra-anal ointment) in the drug label.
Drug Interactions
PDE5 inhibitors
- Phosphodiesterase type 5 (PDE5) inhibitors such as sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates.
- The time course of the interaction appears to be related to the half-life of the PDE5 inhibitor, however, the dose dependence of this interaction has not been studied. Use of RECTIV within a few days of PDE5 inhibitors is contraindicated.
Antihypertensives
- Patients receiving antihypertensive drugs, beta-adrenergic blockers, and other nitrates should be observed for possible additive hypotensive effects when using RECTIV. Marked orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used concomitantly.
- Beta-blockers blunt the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effects. If beta-blockers are used with RECTIV in patients with angina pectoris, additional hypotensive effects may occur.
Aspirin
- Coadministration of aspirin (at doses between 500 mg and 1000 mg) and nitroglycerin has been reported to result in increased nitroglycerin maximum concentrations by as much as 67% and AUC by 73% when administered as a single dose. The pharmacological effects of RECTIV may be enhanced by concomitant administration of aspirin.
Tissue-type Plasminogen Activator (t-PA)
- Intravenous administration of nitroglycerin decreases the thrombolytic effect of tissue-type plasminogen activator (t-PA). Plasma levels of t-PA are reduced when coadministered with nitroglycerin. Therefore, caution should be observed in patients receiving RECTIV during t-PA therapy.
Heparin
- Although an interaction has been reported between intravenous heparin and intravenous nitroglycerin (resulting in a decrease in the anticoagulant effect of heparin), the data are not consistent. If patients are to receive intravenous heparin and RECTIV concurrently, the anticoagulation status of the patient must be checked.
Ergotamine
- Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and consequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore the possibility of ergotism in patients receiving RECTIV should be considered.
Alcohol
- The vasodilating effects of nitroglycerin have been shown to be additive to the effects observed with alcohol.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Nitroglycerin (Intra-anal ointment) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nitroglycerin (Intra-anal ointment) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance on the use of Nitroglycerin (Intra-anal ointment) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Nitroglycerin (Intra-anal ointment) Administration in the drug label.
Monitoring
There is limited information regarding Monitoring of Nitroglycerin (Intra-anal ointment) in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Nitroglycerin (Intra-anal ointment) and IV administrations.
Overdosage
There is limited information regarding Nitroglycerin (Intra-anal ointment) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Nitroglycerin (Intra-anal ointment) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Nitroglycerin (Intra-anal ointment) Mechanism of Action in the drug label.
Structure
There is limited information regarding Nitroglycerin (Intra-anal ointment) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Nitroglycerin (Intra-anal ointment) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Nitroglycerin (Intra-anal ointment) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Nitroglycerin (Intra-anal ointment) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Nitroglycerin (Intra-anal ointment) in the drug label.
How Supplied
There is limited information regarding Nitroglycerin (Intra-anal ointment) How Supplied in the drug label.
Storage
There is limited information regarding Nitroglycerin (Intra-anal ointment) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nitroglycerin (Intra-anal ointment) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nitroglycerin (Intra-anal ointment) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Nitroglycerin (Intra-anal ointment) in the drug label.
Precautions with Alcohol
Alcohol-Nitroglycerin (Intra-anal ointment) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
®
Look-Alike Drug Names
A® — B®
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.