Nirsevimab: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag=Kosar Doraghi, M.D. [mailto:k.doraghi@yahoo.com] |genericName=Nirsevimab |aOrAn=a |drugClass=respiratory syncytial virus (RSV) F protein‑directed fusion inhibitor |indicationType=prevention |indication=RSV lower respiratory tract disease in: Neonates and infants born during or entering their first RSV season, and children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season |hasBlackBo...") |
(No difference)
|
Latest revision as of 15:46, 1 May 2024
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kosar Doraghi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warnings: Hypersensitivity Reactions
See full prescribing information for complete Boxed Warning.
*Hypersensitivity Reactions Including Anaphylaxis
urticaria, dyspnea, cyanosis, and/or hypotonia. Anaphylaxis has been observed with human immunoglobulin G1 (IgG1) monoclonal antibodies. If signs and symptoms of anaphylaxis or other clinically significant hypersensitivity reactions occur, initiate appropriate treatment.
|
Overview
Nirsevimab is a respiratory syncytial virus (RSV) F protein‑directed fusion inhibitor that is FDA approved for the prevention of RSV lower respiratory tract disease in: Neonates and infants born during or entering their first RSV season, and children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash and injection site reactions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Nirsevimab FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
For neonates and infants born during or entering the RSV season, administer BEYFORTUS starting from birth.
- Less than 5 kg: 50 mg by IM injection
- 5 kg and greater: 100 mg by IM injection
- Children who remain vulnerable through their second RSV season:
200 mg (2 x 100 mg injections)
Off-Label Use and Dosage (Pediatric)
Contraindications
infants and children with a history of serious hypersensitivity reactions, including anaphylaxis, to nirsevimab-alip or to any of the excipients
Warnings
|
Warnings: Hypersensitivity Reactions
See full prescribing information for complete Boxed Warning.
*Hypersensitivity Reactions Including Anaphylaxis
urticaria, dyspnea, cyanosis, and/or hypotonia. Anaphylaxis has been observed with human immunoglobulin G1 (IgG1) monoclonal antibodies. If signs and symptoms of anaphylaxis or other clinically significant hypersensitivity reactions occur, initiate appropriate treatment.
|
Hypersensitivity Reactions Including Anaphylaxis: Serious hypersensitivity reactions have been reported following BEYFORTUS administration. These reactions included urticaria, dyspnea, cyanosis, and/or hypotonia. Anaphylaxis has been observed with human immunoglobulin G1 (IgG1) monoclonal antibodies. If signs and symptoms of anaphylaxis or other clinically significant hypersensitivity reactions occur, initiate appropriate treatment.
Adverse Reactions
Clinical Trials Experience
Due to the variability in clinical trial conditions, adverse reaction rates observed in trials for one drug may not directly compare to those of another drug or reflect real-world rates. In combined Phase 2 and Phase 3 trials of BEYFORTUS, 3,224 pediatric subjects received the recommended dose, including 2,119 infants born at 35 weeks gestational age or older and 1,105 infants born earlier. Additionally, 247 infants with specific conditions received BEYFORTUS in Trial 05.
- In Trial 03, conducted on preterm infants born between 29 to less than 35 weeks, subjects were randomized to receive BEYFORTUS or placebo. Trial 04, on late preterm and term infants born at 35 weeks or more, also compared BEYFORTUS to placebo. Infants weighing less than 5 kg received a 50 mg IM dose, while those weighing 5 kg or more received 100 mg.
Pooling data from Trials 03 and 04, the safety of BEYFORTUS was evaluated against placebo. The Safety Population included infants born at various gestational ages and demographics. Adverse reactions were reported in 1.2% of subjects who received BEYFORTUS, with most being mild to moderate.
- In Trial 05 ( Infants Born at <35 Weeks Gestational Age and Infants and Children with CLD of Prematurity or Hemodynamically Significant CHD. BEYFORTUS’s safety was assessed in infants at high risk for severe RSV disease, compared to palivizumab. Infants received BEYFORTUS or palivizumab in a 2:1 ratio. The study included various gestational ages, with subjects receiving BEYFORTUS based on weight. Adverse reactions were similar to previous trials.
For the second RSV season, subjects with CLD or CHD could continue in the trial. Those initially receiving BEYFORTUS continued, while those on palivizumab were re-randomized. Safety data showed consistency with the first season.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of BEYFORTUS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Drug Interactions
Interference with RT-PCR or Rapid Antigen Detection RSV Diagnostic Assays Nirsevimab-alip does not interfere with reverse transcriptase polymerase chain reaction (RT-PCR) or rapid antigen detection RSV diagnostic assays that employ commercially available antibodies targeting antigenic site I, II, or IV on the RSV fusion (F) protein. For immunological assay results which are negative when clinical observations are consistent with RSV infection, it is recommended to confirm using an RT-PCR-based assay.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
BEYFORTUS is not indicated for use in females of reproductive potential.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nirsevimab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nirsevimab during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Nirsevimab in women who are nursing.
Pediatric Use
The safety and effectiveness of BEYFORTUS have been established for the prevention of RSV lower respiratory tract disease in neonates and infants born during or entering their first RSV season and in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
Geriatic Use
There is no FDA guidance on the use of Nirsevimab in geriatric settings.
Gender
There is no FDA guidance on the use of Nirsevimab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nirsevimab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nirsevimab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nirsevimab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nirsevimab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nirsevimab in patients who are immunocompromised.
Administration and Monitoring
Administration
BEYFORTUS should only be administered by a healthcare provider. Before use, visually inspect the solution for any signs of cloudiness, discoloration, or foreign particles. Do not inject if any abnormalities are present. Ensure the pre-filled syringe is undamaged and within its expiration date. Two doses are available: 50 mg and 100 mg, so verify the correct product before administration.
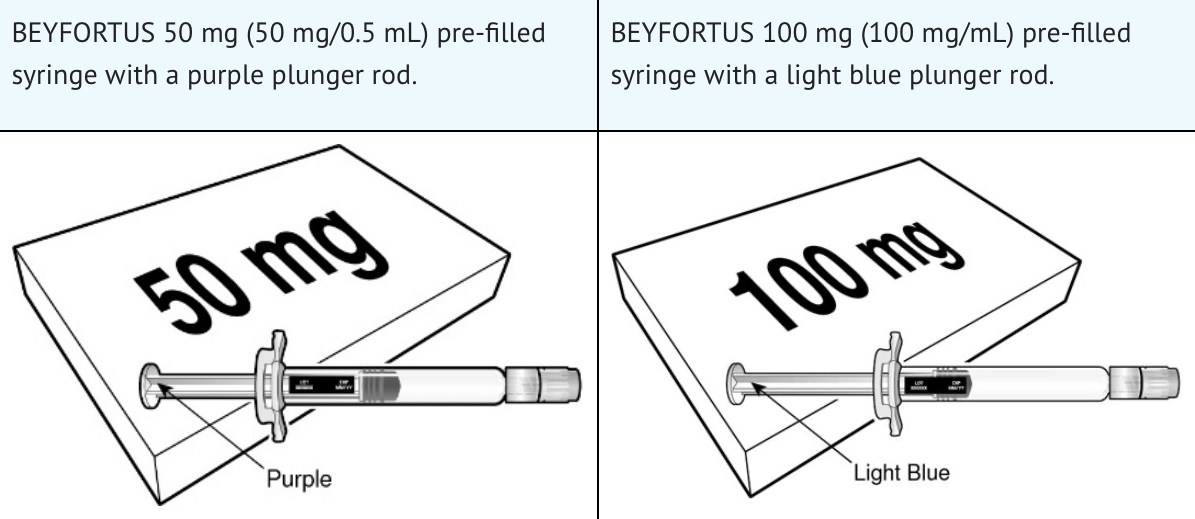 When co-administering with childhood vaccines, use separate syringes and injection sites. Do not mix BEYFORTUS with vaccines or medications. There’s no data on co-administration with other immunoglobulin products. Palivizumab should not be given if BEYFORTUS has already been administered in the same season. The substitution of BEYFORTUS for palivizumab is not recommended once palivizumab treatment has started. BEYFORTUS can be administered to vulnerable children up to 24 months of age before or during their second RSV season.
When co-administering with childhood vaccines, use separate syringes and injection sites. Do not mix BEYFORTUS with vaccines or medications. There’s no data on co-administration with other immunoglobulin products. Palivizumab should not be given if BEYFORTUS has already been administered in the same season. The substitution of BEYFORTUS for palivizumab is not recommended once palivizumab treatment has started. BEYFORTUS can be administered to vulnerable children up to 24 months of age before or during their second RSV season.
Monitoring
There is limited information regarding Nirsevimab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Nirsevimab and IV administrations.
Overdosage
There is limited information regarding Nirsevimab overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Nirsevimab Pharmacology in the drug label.
Mechanism of Action
BEYFORTUS is a monoclonal antibody with anti-RSV activity
Structure
Nirsevimab-alip, a respiratory syncytial virus F protein-directed fusion inhibitor, is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody produced in Chinese hamster ovary (CHO) cells by recombinant DNA technology. The molecular weight is approximately 146.3 kDa. BEYFORTUS (nirsevimab-alip) injection is a sterile, preservative-free, clear to opalescent, colorless to yellow solution for intramuscular injection. It is supplied in a single-dose siliconized Luer lock Type I glass pre-filled syringe with a FluroTec coated plunger stopper. Each 0.5 mL contains 50 mg nirsevimab-alip, arginine hydrochloride (8 mg), histidine (1.1 mg), L-histidine hydrochloride monohydrate (1.6 mg), polysorbate 80 (0.1 mg), sucrose (21 mg), and water for injection (USP). The pH is 6.0. Each 1 mL contains 100 mg nirsevimab-alip, arginine hydrochloride (17 mg), histidine (2.2 mg), L-histidine hydrochloride monohydrate (3.3 mg), polysorbate 80 (0.2 mg), sucrose (41 mg), and water for injection (USP). The pH is 6.0.
Pharmacodynamics
There is a positive correlation between a serum nirsevimab-alip AUC (based on clearance at baseline) above 12.8 mg*day/mL and a lower incidence of medically attended RSV lower respiratory tract infection (MA RSV LRTI). Following IM administration of nirsevimab-alip in adults, RSV neutralizing antibody levels in serum were approximately 4 times higher than baseline at 8 hours after nirsevimab-alip dosing, and maximum levels were reached by day 6 following IM administration of nirsevimab-alip in adults. The safety and effectiveness of BEYFORTUS have not been established in adults. Duration of Protection Based on clinical data, the duration of protection offered by a single dose of BEYFORTUS extends through 5 months.
Pharmacokinetics
Nirsevimab-alip’s PK is dose-proportional across pediatric subjects, ranging from 25 mg to 200 mg in a single IM administration. Following the recommended dose, serum exposures are consistent across different populations, including neonates, infants born prematurely, and pediatric subjects with CLD or CHD.
- Absorption:
The absolute bioavailability of nirsevimab-alip is estimated at 84%, with a median time to maximum concentration of 6 days.
- Microbiology
Mechanism of Action:Nirsevimab-alip, a recombinant human IgG1κ monoclonal antibody, targets the prefusion conformation of the RSV F protein, providing passive immunity. Its long-acting nature is due to a triple amino acid substitution (YTE) in the Fc region, enhancing binding to the neonatal Fc receptor and extending serum half-life. By binding to a conserved epitope on the prefusion protein, it neutralizes RSV by preventing conformational changes necessary for viral entry. Antiviral Activity:In cell culture, nirsevimab-alip effectively neutralizes RSV A and B strains collected globally from 2003 to 2017. Antiviral Resistance:Escape variants selected in cell culture exhibit reduced susceptibility to nirsevimab-alip, with associated substitutions in the binding site of the RSV F protein. However, polymorphisms conferring large fold-reductions in susceptibility are rare in surveillance trials. In clinical trials, known resistance-associated substitutions were infrequent, with ongoing testing for novel variants. Cross-resistance:Limited data suggest variants resistant to nirsevimab-alip may have cross-resistance to palivizumab, although palivizumab retained potency against resistance-associated substitutions identified in trials. Immunogenicity:The incidence of anti-drug antibodies (ADA) varied across trials, with most ADA targeting the YTE substitution. ADA presence did not significantly affect nirsevimab-alip serum concentrations, but those with ADA had lower concentrations at Day 361. The impact of ADA on BEYFORTUS effectiveness remains uncertain due to low occurrence rates in clinical trials.
Nonclinical Toxicology
There is limited information regarding Nirsevimab Nonclinical Toxicology in the drug label.
Clinical Studies
The efficacy and safety of BEYFORTUS were evaluated in term and preterm infants in the trials summarized
- Prevention of MA RSV LRTI in Infants Born at ≥29 to <35 Weeks Gestational Age (Trial 03)
Trial 03 was a randomized, double-blind, placebo-controlled multicenter trial aimed at preventing Medically Attended Respiratory Syncytial Virus Lower Respiratory Tract Infection (MA RSV LRTI) in preterm infants born between ≥29 and <35 weeks gestational age (GA). Subjects were randomized 2:1 to receive BEYFORTUS (N=969) or placebo (N=484) via IM injection, with all BEYFORTUS recipients receiving a 50 mg IM dose. The recommended BEYFORTUS dose for neonates and infants during their first RSV season is a single IM dose of 50 mg or 100 mg for those weighing less than 5 kg or ≥5 kg, respectively.
- At randomization, subjects had varying gestational ages, with the majority between ≥32 and <35 weeks GA. Demographics included a mix of genders and ethnicities, with most subjects from the Northern Hemisphere. The median age was 2.8 months, with a distribution across different age groups.
The primary endpoint was the incidence of MA RSV LRTI confirmed by RT-PCR-confirmed RSV, predominantly presenting as bronchiolitis or pneumonia within 150 days post-dosing. Medically Attended visits encompassed healthcare provider visits, including physician offices, urgent care, emergency room visits, and hospitalizations. Signs of LRTI involvement included specific respiratory symptoms, along with indications of worsening clinical severity. The incidence of RSV LRTI requiring hospitalization was a secondary endpoint, defined as hospitalization for LRTI with a positive RSV test.
- Prevention of MA RSV LRTI in Infants Born at ≥35 Weeks Gestational Age (Trial 04)
BEYFORTUS underwent evaluation in a Phase 3 randomized, double-blind, placebo-controlled multicenter trial, Trial 04, targeting the prevention of Medically Attended Respiratory Syncytial Virus Lower Respiratory Tract Infection (MA RSV LRTI) in term and late preterm infants with a gestational age (GA) of ≥35 weeks entering their first RSV season. The primary analysis population, consisting of 1,490 term and late preterm infants (GA ≥35 weeks), were randomized 2:1 to receive a single IM dose of BEYFORTUS (N=994) or placebo (N=496). Demographic distribution included varying gestational ages, genders, ethnicities, and geographical origins, with the majority being from the Northern Hemisphere. The median age was 2.6 months, with a distribution across different age groups. The primary endpoint was the incidence of MA RSV LRTI confirmed by RT-PCR-confirmed RSV, aligned with Trial 03’s definition. Additionally, the incidence of RSV LRTI requiring hospitalization was a prespecified secondary endpoint, defined as hospitalization for LRTI with a positive RSV test.
- Prevention of MA RSV LRTI in Infants Born at <35 Weeks Gestational Age and Infants with CLD of Prematurity or Hemodynamically Significant CHD (Trial 05)
The safety and pharmacokinetics (PK) of BEYFORTUS were assessed in a Phase 2/3 randomized, double-blind, palivizumab-controlled multicenter trial (Trial 05) involving pediatric subjects born less than 35 weeks gestational age (GA) and infants with chronic lung disease (CLD) of prematurity or hemodynamically significant congenital heart disease (CHD). While not primarily designed for efficacy, BEYFORTUS’s efficacy was explored as a secondary endpoint. Efficacy was extrapolated from Trials 03 and 04 based on similar nirsevimab-alip exposures among subjects enrolled in Trial 04 and 05. In Trial 05’s first RSV season, 925 infants were randomized, with 616 in the BEYFORTUS group and 309 in the palivizumab group. The incidence of MA RSV LRTI through 150 days post-dose was 0.6% in the BEYFORTUS group and 1.0% in the palivizumab group. During the second RSV season, pediatric subjects with CLD of prematurity or hemodynamically significant CHD continued in the trial (n=262). No cases of MA RSV LRTI were reported through Day 150 post-dose in subjects who received either BEYFORTUS or palivizumab.
How Supplied
- One 50 mg/0.5 mL single-dose pre-filled syringe in a carton: NDC 49281-575-00
- Five 50 mg/0.5 mL single-dose pre-filled syringes in a carton: NDC 49281-575-15
- One 100 mg/mL single-dose pre-filled syringe in a carton: NDC 49281-574-88
- Five 100 mg/mL single-dose pre-filled syringes in a carton: NDC 49281-574-15
Each BEYFORTUS pre-filled syringe is for one time use only.
Storage
Store refrigerated between 36°F to 46°F (2°C to 8°C). BEYFORTUS may be kept at room temperature 68°F to 77°F (20°C to 25°C) for a maximum of 8 hours. After removal from the refrigerator, BEYFORTUS must be used within 8 hours or discarded. Store BEYFORTUS in original carton to protect from light until time of use.
Images
Drug Images
{{#ask: Page Name::Nirsevimab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
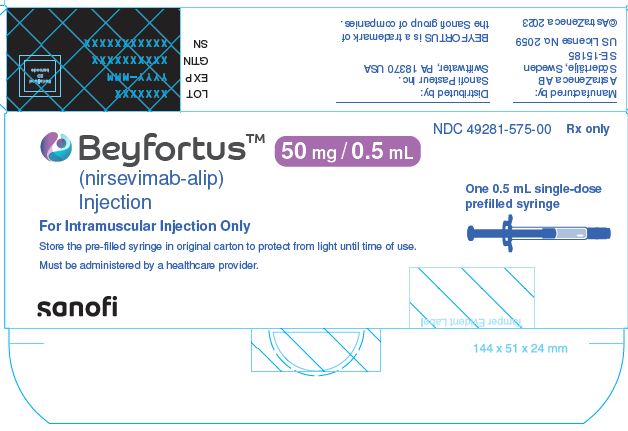
NDC 49281-575-00 Rx only
Beyfortus™ (nirsevimab-alip) Injection 50mg/0.5mL
One 0.5 mL single-dose prefilled syringe
For Intramuscular Injection Only
Store the pre-filled syringe in original carton to protect from light until time of use.
Must be administered by a healthcare provider.
sanofi
 {{#ask: Label Page::Nirsevimab
|?Label Name
|format=template
|template=DrugLabelImages
|mainlabel=-
|sort=Label Page
}}
{{#ask: Label Page::Nirsevimab
|?Label Name
|format=template
|template=DrugLabelImages
|mainlabel=-
|sort=Label Page
}}
Patient Counseling Information
There is limited information regarding Nirsevimab Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Nirsevimab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Nirsevimab Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Nirsevimab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.