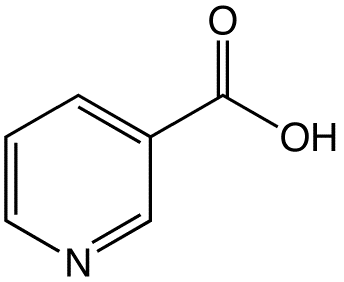
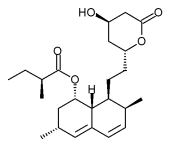
Niacin/lovastatin
 | |
 | |
| Combination of | |
|---|---|
| Niacin | n/a |
| Lovastatin | Statin |
| [[{{{component3}}}]] | ? Class |
| [[{{{component4}}}]] | ? Class |
| [[{{{component5}}}]] | ? Class |
| Clinical data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Niacin/lovastatin (tradename Advicor) is a drug combination used for the treatment of dyslipidemia. It is a combination of niacin and the statin drug lovastatin (best known as Mevacor in the U.S.). The combination preparation is marketed by Abbott Laboratories
It was approved by the FDA on December 17, 2001.
Dosage
The combination is available as tablets containing:.[1]
- 500 mg/20 mg
- 750 mg/20 mg
- 1000 mg/20 mg
- 1000 mg/40 mg
External links
- Advicor website
- ↑ "Advicor package insert, How Supplied section". Retrieved 2008-03-15.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Drugs that are a combination of chemicals
- Drugs