Neuroendocrine tumors: Difference between revisions
Sara Mohsin (talk | contribs) |
Sara Mohsin (talk | contribs) No edit summary |
||
| (119 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Neuroendocrine tumors}} | {{Neuroendocrine tumors}} | ||
'''For patient information, click [[Neuroendocrine tumors (patient information)|here]]''' | '''For patient information, click [[Neuroendocrine tumors (patient information)|here]]''' | ||
{{CMG}} {{AE}}{{S.M.}} | {{CMG}} {{AE}} {{S.M.}} | ||
==Overview== | ==Overview== | ||
| Line 25: | Line 10: | ||
==Historical Perspective== | ==Historical Perspective== | ||
* In 1907, Siegfried Oberndorfer was the first person to clearly distinguish GEP-NETs from other forms of cancer. Since they were so slow-growing, he considered them to be "cancer-like" rather than truly cancerous, and hence, he coined the term "carcinoid" for these tumors. | * In 1907, Siegfried Oberndorfer was the first [[person]] to clearly [[Distinctive feature|distinguish]] GEP-[[NET1|NETs]] from other forms of [[cancer]]. Since they were so [[slow]]-[[Growth|growing]], he considered them to be "[[cancer]]-like" rather than truly [[cancerous]], and hence, he coined the [[Term logic|term]] "[[carcinoid]]" for these [[Tumor|tumors]]. | ||
* In 1929, Siegfried Oberndorfer reported that some such tumors were not so indolent and distinguished them as PETs (mostly called carcinoids). Despite of the differences between the two categories, even in the twenty-first century, some doctors (including oncologists) insist on calling all GEP-NETs "carcinoid". | * In 1929, Siegfried Oberndorfer reported that some such [[tumors]] were not so indolent and [[Distinctive feature|distinguished]] them as [[PET|PETs]] (mostly called [[Carcinoid|carcinoids]]). Despite of the [[Difference (philosophy)|differences]] between the two [[categories]], even in the twenty-first [[century]], some [[doctors]] (including [[oncologists]]) insist on calling all GEP-[[NET1|NETs]] "[[carcinoid]]". | ||
*In 1988, the earliest synthetic form of somatostatin used in the treatment of | *In 1988, the earliest [[Synthetic element|synthetic]] form of [[somatostatin]] [[Usage analysis|used]] in the [[Treatment Planning|treatment]] of [[neuroendocrine]] [[tumors]] was [[octreotide]], which was first [[Market basket|marketed]] by [[Sandoz]] as [[Sandostatin]]. | ||
*In 2006 and 2007, the European Neuroendocrine | *In 2006 and 2007, the European [[Neuroendocrine]] [[Tumor]] Society (ENETS) [[Proposition|proposed]] a [[Staging (pathology)|staging]] scheme same as for most other types of [[epithelial]] [[neoplasms]] for GEP NENs, alongwith a [[histologic]] [[Grading (tumors)|grading]] [[system]] [[Applicability Domain|applicable]] to all [[disease]] [[Stages of human development|stages]]. [[American Joint Committee on Cancer]] (AJCC) and the [[UICC|Union for International Cancer Control (UICC)]] later on endorsed this [[Grading (tumors)|grading]] [[Proposition|proposal]] for the [[tumor]], [[Nodal (protein)|node]], [[metastasis]] ([[TNM classification|TNM]]) [[Staging (pathology)|staging]] [[classification]] of [[digestive system]] NENs, after [[Modifications (genetics)|modifying]] the [[Cancer staging|staging]] [[Parameter|parameters]] of the ENETS [[Proposition|proposal]]. | ||
*Th 2010 WHO classification of tumors of the gastrointestinal tract, liver, and pancreas (which was subsequently updated in 2017) also endorsed the ENETS grading scheme for NENs of the digestive tract, separating well-differentiated tumors into low-grade (G1) and intermediate-grade (G2) categories. All poorly differentiated NETs are high-grade (G3) NECs according to this classification scheme. | *Th 2010 [[World Health Organization|WHO]] [[classification]] of [[tumors]] of the [[gastrointestinal tract]], [[liver]], and [[pancreas]] (which was subsequently updated in 2017) also endorsed the ENETS [[Grading (tumors)|grading]] scheme for NENs of the [[digestive tract]], [[Separation process|separating]] [[WellPoint|well]]-[[Differentiate|differentiated]] [[tumors]] into low-[[Grading (tumors)|grade]] ([[G1]]) and intermediate-[[Grading (tumors)|grade]] ([[G2 phase|G2]]) [[categories]]. All poorly [[Differentiate|differentiated]] [[NET1|NETs]] are high-[[Grading (tumors)|grade]] (G3) NECs according to this [[classification]] scheme. | ||
==Classification== | ==Classification== | ||
| Line 38: | Line 23: | ||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Percentage}} | !style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Percentage}} | ||
|- | |- | ||
| rowspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold"|'''Carcinoids (about two thirds of GEP-NETs)''' | | rowspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold"|'''[[Carcinoid|Carcinoids]] (about two thirds of GEP-[[NET1|NETs]])''' | ||
| | | | ||
*With carcinoid syndrome | *With [[carcinoid syndrome]] | ||
| | | | ||
* About 10 percent of carcinoids | * About 10 [[Percentage|percent]] of [[Carcinoid|carcinoids]] | ||
|- | |- | ||
| | | | ||
* Without carcinoid syndrome | * Without [[carcinoid syndrome]] | ||
| | | | ||
* About 90 percent of carcinoids | * About 90 [[Percentage|percent]] of [[Carcinoid|carcinoids]] | ||
|- | |- | ||
| rowspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold"|'''PETs (about one third of GEP-NETs)''' | | rowspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold"|'''[[PET|PETs]] (about one third of GEP-[[NET1|NETs]])''' <br /> | ||
| | | | ||
* Nonfunctioning | * Nonfunctioning | ||
| | | | ||
* 15 to 30 percent of PETs | * 15 to 30 [[Percentage|percent]] of [[PET|PETs]] | ||
|- | |- | ||
| | | | ||
* Functioning: | *[[Function (biology)|Functioning]]: | ||
** [[Gastrinoma]] (produces excessive [[gastrin]] and causes [[Zollinger-Ellison Syndrome]] [ZES]) | **[[Gastrinoma]] ([[Product (biology)|produces]] [[Excess dose|excessive]] [[gastrin]] and [[causes]] [[Zollinger-Ellison Syndrome]] [<nowiki/>[[Zollinger-Ellison syndrome|ZES]]]) | ||
**[[Insulinoma]] (produces excessive [[insulin]]) | **[[Insulinoma]] ([[Product (biology)|produces]] [[Excess risk|excessive]] [[insulin]]) | ||
**[[Glucagonoma]] (produces excessive [[glucagon]]) | **[[Glucagonoma]] ([[Product (biology)|produces]] [[Excess risk|excessive]] [[glucagon]]) | ||
**[[Vasoactive intestinal peptideoma]] ([[VIPoma]]) (produces excessive [[vasoactive intestinal peptide]] [VIP]) | **[[Vasoactive intestinal peptideoma]] ([[VIPoma]]) ([[Product (biology)|produces]] excessive [[vasoactive intestinal peptide]] [<nowiki/>[[VIP]]]) | ||
**[[PPoma]] (produces excessive [[pancreatic polypeptide]] [often classed with nonfunctioning PETs]) | **[[PPoma]] ([[Product (biology)|produces]] excessive [[pancreatic polypeptide]] [often [[Class (biology)|classed]] with nonfunctioning [[PET|PETs]]]) | ||
**[[Somatostatinoma]] (produces excessive [[somatostatin]]) | **[[Somatostatinoma]] ([[Product (biology)|produces]] excessive [[somatostatin]]) | ||
**[[Watery diarrhea]], [[hypokalemia]]-[[achlorhydria]] (WDHA) | **[[Watery diarrhea]], [[hypokalemia]]-[[achlorhydria]] ([[WDHA]]) | ||
**[[CRHoma]] (produces excessive [[ | **[[CRHoma]] ([[Product (biology)|produces]] excessive [[Corticotropin-releasing hormone|corticotropin-releasing hormones]] [<nowiki/>[[CRH]]]) | ||
**[[Calcitoninoma]] (produces excessive [[calcitonin]]) | **[[Calcitoninoma]] ([[Product (biology)|produces]] excessive [[calcitonin]]) | ||
**[[GHRHoma]] (produces excessive [[GHRH|growth-hormone-releasing hormone]] [GHRH]) | **[[GHRHoma]] ([[Product (biology)|produces]] excessive [[GHRH|growth-hormone-releasing hormone]] [<nowiki/>[[GHRH]]]) | ||
**[[Neurotensinoma]] (produces excessive [[neurotensin]]) | **[[Neurotensinoma]] ([[Product (biology)|produces]] excessive [[neurotensin]]) | ||
**[[ACTHoma]] (produces excessive [[adrenocorticotropic hormone]] [ACTH]) | **[[ACTHoma]] ([[Product (biology)|produces]] excessive [[adrenocorticotropic hormone]] [<nowiki/>[[ACTH]]]) | ||
**[[GRFoma]] (produces excessive [[growth hormone]] | **[[GRFoma]] ([[Product (biology)|produces]] excessive [[Growth hormone-releasing factor|growth hormone release factor]] [<nowiki/>[[GRF]]]) | ||
**[[Parathyroid hormone–related peptide tumor]] | **[[Parathyroid hormone–related peptide tumor|Parathyroid hormone-related peptide tumor]] | ||
| | | | ||
* 70 to 85 percent of PETs | * 70 to 85 [[Percentage|percent]] of [[PET|PETs]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Rare GEP-NETs''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Rare GEP-[[NET1|NETs]]''' | ||
| | | | ||
*Medullary carcinoma of the | *[[Medullary carcinoma of the thyroid]] | ||
*[[Merkel cell cancer]] (trabecular cancer) | *[[Merkel cell cancer]] ([[trabecular cancer]]) | ||
*[[Small-cell lung cancer]] (SCLC) | *[[Small-cell lung cancer]] ([[Small cell lung cancer|SCLC]]) | ||
* Large-cell neuroendocrine carcinoma (of the lung) | *[[Large-cell neuroendocrine carcinoma]] (of the [[lung]]) | ||
* Neuroendocrine carcinoma of the [[cervix]] | *[[Neuroendocrine]] [[carcinoma]] of the [[cervix]] | ||
* Multiple Endocrine Neoplasia type 1 (MEN-1 or MEN1) (usually nonfunctioning) (also causing ZES) | *[[Multiple endocrine neoplasia type 1|Multiple Endocrine Neoplasia type 1]] ([[MEN1|MEN-1]] or [[MEN1]]) (usually nonfunctioning) (also [[Causes|causing]] ZES) | ||
* Multiple Endocrine Neoplasia type 2 (MEN-2 or MEN2) | *[[Multiple endocrine neoplasia type 2|Multiple Endocrine Neoplasia type 2]] ([[MEN2|MEN-2]] or [[MEN2]]) | ||
*[[Neurofibromatosis type 1]] | *[[Neurofibromatosis type 1]] | ||
*[[Tuberous sclerosis]] | *[[Tuberous sclerosis]] | ||
*[[Von Hippel-Lindau disease]] | *[[Von Hippel-Lindau disease]] ([[VHL]]) | ||
*[[Neuroblastoma]] | *[[Neuroblastoma]] | ||
*[[Pheochromocytoma]] (phaeochromocytoma) | *[[Pheochromocytoma]] ([[phaeochromocytoma]]) | ||
*[[Paraganglioma]] | *[[Paraganglioma]] | ||
* Neuroendocrine tumor of the | *[[Neuroendocrine]] [[tumor]] of the [[Anterior pituitary gland|anterior pituitary]] | ||
* Carney's complex | *[[Carney complex|Carney's complex]] | ||
| | | | ||
|} | |} | ||
| Line 99: | Line 84: | ||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Name/type of neuroendocrine tumor}} | !style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Name/type of neuroendocrine tumor}} | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Pituitary gland | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Pituitary gland]] | ||
| | | | ||
* Neuroendocrine tumor of the anterior pituitary | *[[Neuroendocrine]] [[tumor]] of the [[anterior pituitary]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Thyroid gland | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Thyroid gland]] | ||
| | | | ||
* Neuroendocrine thyroid tumors | *[[Neuroendocrine]] [[Thyroid cancer|thyroid tumors]] | ||
** Medullary thyroid carcinoma (particularly) | **[[Medullary thyroid carcinoma]] (particularly) | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Parathyroid glands | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Parathyroid glands]] | ||
| | | | ||
* Parathyroid tumors | *[[Parathyroid]] [[tumors]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Thymus and mediastinum | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Thymus]] and [[mediastinum]] | ||
| | | | ||
* Thymus and mediastinal carcinoid tumors | *[[Thymus cancer|Thymus]] and [[mediastinal]] [[Carcinoid Tumor|carcinoid tumors]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Lungs | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Lungs]] | ||
| | | | ||
* Pulmonary neuroendocrine tumors | *[[Pulmonary]] [[neuroendocrine]] [[tumors]] | ||
** Bronchus | **[[Bronchus]] | ||
** Pulmonary carcinoid tumors: | **[[Pulmonary]] [[carcinoid tumors]]: | ||
*** Typical carcinoid (TC; low-grade) | ***[[Typical set|Typical]] [[carcinoid]] (TC; low-[[Grading (tumors)|grade]]) | ||
*** Atypical carcinoid (AC; intermediate-grade) | *** Atypical [[carcinoid]] (AC; intermediate-[[Grading (tumors)|grade]]) | ||
** Small-cell lung cancer (SCLC) | **[[Small-cell lung cancer]] ([[SCLC]]) | ||
** Large cell neuroendocrine carcinoma of the lung (LCNEC) | **[[Large cell neuroendocrine carcinoma of the lung]] ([[LCNEC]]) | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Extrapulmonary | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Extrapulmonary | ||
| | | | ||
* Extrapulmonary small cell carcinomas (ESCC or EPSCC) | * Extrapulmonary [[small cell]] [[carcinomas]] (ESCC or EPSCC) | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |GIT | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Gastrointestinal tract|GIT]] | ||
| | | | ||
* Gastroenteropancreatic neuroendocrine tumors (GEP-NET) | * Gastroenteropancreatic [[neuroendocrine]] [[tumors]] (GEP-[[NET1|NET]]) | ||
** Foregut GEP-NET (foregut tumors can conceptually | **[[Foregut]] GEP-[[NET1|NET]] ([[foregut]] [[tumors]] can [[Conceptual clustering|conceptually]] encompass not only [[NET1|NETs]] of the [[stomach]] and [[proximal]] [[duodenum]], but also the [[pancreas]], and even [[thymus]], [[lung]] and [[bronchus]]) | ||
*** Pancreatic endocrine tumors (if considered separately from foregut GEP-NET) | ***[[Pancreatic]] [[endocrine tumors]] (if considered separately from [[foregut]] GEP-[[NET1|NET]]) | ||
** Midgut GEP-NET (from distal half of 2nd part of the duodenum to the proximal two-thirds of the transverse colon) | **[[Midgut]] GEP-[[NET1|NET]] (from [[distal]] half of 2nd part of the [[duodenum]] to the [[proximal]] two-thirds of the [[transverse colon]]) | ||
*** | ***[[Appendix]], including well-[[Differentiate|differentiated]] [[NET1|NETs]] ([[benign]]); well-[[Differentiate|differentiated]] [[NET1|NETs]] (uncertain [[malignant]] [[potential]]); well-[[Differentiate|differentiated]] [[neuroendocrine]] [[carcinoma]] (with low [[malignant]] [[potential]]); [[Mixed-handed|mixed]] [[exocrine]]-[[neuroendocrine]] [[carcinoma]] ([[Goblet cell tumor of appendix|goblet cell carcinoma]], also called adenocarcinoid and [[mucous]] adenocarcinoid) | ||
** Hindgut GEP-NET | **[[Hindgut]] GEP-[[NET1|NET]] | ||
* Liver and gallbladder | *[[Liver]] and [[gallbladder]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Adrenal gland | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Adrenal gland]] | ||
| | | | ||
* Adrenal tumors, particularly adrenomedullary tumors | *[[Adrenal tumor|Adrenal tumors]], particularly [[adrenomedullary]] [[tumors]] | ||
* Pheochromocytoma | *[[Pheochromocytoma]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Peripheral nervous system | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Peripheral nervous system]] | ||
|Peripheral nervous system tumors, such as: | |[[Peripheral nervous system]] [[tumors]], such as: | ||
* Schwannoma | |||
* Paraganglioma | *[[Schwannoma]] | ||
* Neuroblastoma | *[[Paraganglioma]] | ||
*[[Neuroblastoma]] | |||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Mammary gland | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Mammary gland]] | ||
| | | | ||
* Breast tumors | *[[Breast cancer|Breast tumors]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Genitourinary tract | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Genitourinary tract]] | ||
| | | | ||
* Urinary tract carcinoid tumor and neuroendocrine carcinoma | *[[Urinary tract]] [[carcinoid tumor]] and [[neuroendocrine]] [[carcinoma]] | ||
* Ovary | *[[Ovary]] | ||
* Neuroendocrine tumor of the cervix | *[[Neuroendocrine]] [[tumor]] of the [[cervix]] | ||
* Testes | *[[Testes]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Skin | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Skin]] | ||
| | | | ||
* Merkel cell carcinoma of skin (trabecular cancer) | *[[Merkel cell carcinoma]] of the [[skin]] ([[trabecular cancer]]) | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Multiple organs involvement in inherited conditions | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Multiple [[organs]] involvement in [[inherited]] [[conditions]] | ||
| | | | ||
* Several inherited conditions: | * Several [[inherited]] [[conditions]]: | ||
** Multiple endocrine neoplasia type 1 (MEN1) | **[[Multiple endocrine neoplasia type 1]] ([[MEN1]]) | ||
** Multiple endocrine neoplasia type 2 (MEN2) | **[[Multiple endocrine neoplasia type 2]] ([[MEN2]]) | ||
** Von Hippel-Lindau (VHL) disease | **[[Von Hippel-Lindau Disease|Von Hippel-Lindau (VHL) disease]] | ||
** Neurofibromatosis type 1 | **[[Neurofibromatosis type 1]] | ||
** Tuberous sclerosis | **[[Tuberous sclerosis]] | ||
** Carney complex | **[[Carney complex]] | ||
|} | |} | ||
===Classification of GEP-NETs by cell characteristics=== | ===Classification of GEP-NETs by cell characteristics=== | ||
* The diverse and amorphous nature of GEP-NETs has led to a confused, overlapping, and changing terminology. | * The diverse and [[amorphous]] [[nature]] of GEP-[[NET1|NETs]] has led to a [[Confusion|confused]], overlapping, and [[Change detection|changing]] [[Term logic|terminology]]. | ||
* In general, aggressiveness (malignancy), secretion (of hormones), and anaplasia (dissimilarity between tumor cells and normal cells) tend to go together, but there are many exceptions, which have contributed to the confusion in terminology. For example, the term | * In [[General Relativity|general]], [[aggressiveness]] ([[malignancy]]), [[secretion]] (of [[hormones]]), and [[anaplasia]] (dissimilarity between [[Tumor cell|tumor cells]] and [[normal]] [[Cells (biology)|cells]]) tend to go together, but there are many exceptions, which have contributed to the [[confusion]] in [[Term logic|terminology]]. [[Example 1|For example]], the [[Term logic|term]] atypical [[carcinoid]] is sometimes used to [[Indication (medicine)|indicate]] an aggressive [[tumor]] without [[secretions]], whether [[anaplastic]] or well-[[Differentiate|differentiated]]. | ||
* In 2000, the World Health Organization (WHO) revised the classification of GEP-NETs, abandoning the term | * In 2000, the [[World Health Organization]] ([[World Health Organization|WHO]]) revised the [[classification]] of GEP-[[NET1|NETs]], abandoning the [[Term logic|term]] [[carcinoid]] in favor of [[neuroendocrine]] [[tumor]] ([[NET1|NET]]) and abandoning [[Islet cell cancer|islet cell tumor]] or [[pancreatic]] [[Endocrine tumors|endocrine tumor]] for [[neuroendocrine]] [[carcinoma]] (NEC). | ||
* Judging from papers published into 2006, the medical community is accepting this new terminology with great sluggishness. (Perhaps one reason for the resistance is that the WHO chose to label the least aggressive subclass of neuroendocrine neoplasm with the term – | * Judging from papers published into 2006, the [[medical]] community is [[Acceptor|accepting]] this [[new]] [[Term logic|terminology]] with great [[sluggishness]]. (Perhaps one [[Reasoning|reason]] for the [[resistance]] is that the [[World Health Organization|WHO]] chose to [[label]] the least aggressive [[Subclass (biology)|subclass]] of [[neuroendocrine]] [[neoplasm]] with the [[Term logic|term]] – [[neuroendocrine]] [[tumor]] – [[Wide and fast|widely]] [[Usage analysis|used]] previously either for the [[Superclass (biology)|superclass]] or for the [[General Relativity|generally]] aggressive noncarcinoid [[Subclass (biology)|subclass]]). | ||
* Klöppel ''et alia'' have written an overview that clarifies the WHO classification and bridges the gap to the old terminology (Klöppel, Perren, and Heitz 2004), this old terminology is given in the table below: | * Klöppel ''et alia'' have [[Writing|written]] an [[overview]] that clarifies the [[World Health Organization|WHO]] [[classification]] and [[Bridge (chemical)|bridges]] the gap to the old [[Term logic|terminology]] (Klöppel, Perren, and Heitz 2004), this old [[Term logic|terminology]] is given in the table below: | ||
====Summary of classification by cell characteristics (the WHO classification)==== | ====Summary of classification by cell characteristics (the WHO classification)==== | ||
* GEP-NETs | * This classification was done due to peripheral receptors. | ||
*GEP-[[NET1|NETs]] receptors (''[[APUDoma of skin|APUDomas]]) are the main detected receptors.'' | |||
*That [[Term logic|term]] is now misleading, since it is [[Base|based]] on a discredited [[theory]] of the [[development]] of the [[tumors]]. | |||
*It is now known that this receptor may not be indicator of cell origin.<ref> | |||
"The APUD concept led to the belief that these cells arise from the embryologic neural crest. This hypothesis eventually was found to be incorrect" (Warner 2005, 2). | "The APUD concept led to the belief that these cells arise from the embryologic neural crest. This hypothesis eventually was found to be incorrect" (Warner 2005, 2). | ||
<p> | <p> | ||
| Line 197: | Line 186: | ||
! colspan="5" style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Superclass:Öberg, WHO, Klöppel ''et alia'': Gastro-entero-pancreatic neuroendocrine tumor (GEP-NET)}} | ! colspan="5" style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Superclass:Öberg, WHO, Klöppel ''et alia'': Gastro-entero-pancreatic neuroendocrine tumor (GEP-NET)}} | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Subclass 1 (less malignant)''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Subclass (biology)|Subclass]] 1 (less [[malignant]])''' | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Subclass 2 (more malignant)''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Subclass (biology)|Subclass]] 2 (more [[malignant]])''' | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Subclass 3 (most malignant)''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Subclass (biology)|Subclass]] 3 (most [[malignant]])''' | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Subclass 4 (mixed)''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Subclass (biology)|Subclass]] 4 ([[Mixed-handed|mixed]])''' | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Subclass 5 (miscellaneous)''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Subclass (biology)|Subclass]] 5 (miscellaneous)''' | ||
|- | |- | ||
| | | | ||
*'''Öberg:''' Carcinoid | *'''Öberg:''' [[Carcinoid]] | ||
*'''WHO:''' Neuroendocrine tumor (NET) | *'''[[WHO]]:''' [[Neuroendocrine]] [[tumor]] ([[NET1|NET]]) | ||
*'''Klöppel ''et alia'':''' Well-differentiated neuroendocrine tumor (NET) (carcinoid) | *'''Klöppel ''et alia'':''' Well-[[Differentiate|differentiated]] [[neuroendocrine]] [[tumor]] ([[NET1|NET]]) ([[carcinoid]]) | ||
*''' | *'''This article:''' [[Carcinoid]] | ||
| | | | ||
*'''Öberg:''' Endocrine pancreatic tumor | *'''Öberg:''' [[Endocrine]] [[pancreatic tumor]] | ||
*'''WHO:''' Neuroendocrine carcinoma (NEC) | *'''[[WHO]]:''' [[Neuroendocrine]] [[carcinoma]] (NEC) | ||
*'''Klöppel ''et alia'':''' Well-differentiated neuroendocrine carcinoma (NEC) (malignant carcinoid) | *'''Klöppel ''et alia'':''' Well-[[Differentiate|differentiated]] [[neuroendocrine]] [[carcinoma]] (NEC) ([[malignant]] [[carcinoid]]) | ||
*''' | *'''This article:''' [[Pancreatic]] [[Endocrine tumors|endocrine tumor]] ([[PET]]) or [[endocrine]] [[pancreatic tumor]] ([[EPT]]) or [[Pancreatic islet cell carcinoma|islet cell tumor]] or noncarcinoid GEP-[[NET1|NET]] | ||
| | | | ||
*'''WHO:''' Poorly-differentiated neuroendocrine carcinoma | *'''[[WHO]]:''' Poorly-[[Differentiate|differentiated]] [[neuroendocrine]] [[carcinoma]] | ||
*'''Klöppel ''et alia'':''' Poorly-differentiated neuroendocrine carcinoma (high-grade malignant carcinoid) | *'''Klöppel ''et alia'':''' Poorly-[[Differentiate|differentiated]] [[neuroendocrine]] [[carcinoma]] (high-[[Grading (tumors)|grade]] [[malignant]] [[carcinoid]]) | ||
| | | | ||
* '''WHO:''' Mixed endocrine/exocrine tumor | * '''[[WHO]]:''' [[Mixed-handed|Mixed]] [[endocrine]]/[[exocrine]] [[tumor]] | ||
| | | | ||
* '''WHO:''' Rare neuroendocrine-like lesions | * '''[[WHO]]:''' [[Rare]] [[neuroendocrine]]-like [[lesions]] | ||
|} | |} | ||
===2010 WHO Classification Of NET=== | ===2010 WHO Classification Of NET=== | ||
*2010 WHO Classification | *2010 [[World Health Organization|WHO]] [[Classification]] of [[neuroendocrine]] [[Tumor|tumors]] with their ICD-O-3 [[Code|codes]] is given below: | ||
{| class="wikitable" | {| class="wikitable" | ||
|+2010 World Health Organization International Classification and Distribution of Diseases for Oncology (3rd Ed. (ICD-O-3) Codes of Neuroendocrine Tumors (NETs) in the Gastrointestinal and Pancreatobiliary Tracts) | |+2010 World Health Organization International Classification and Distribution of Diseases for Oncology (3rd Ed. (ICD-O-3) Codes of Neuroendocrine Tumors (NETs) in the Gastrointestinal and Pancreatobiliary Tracts) | ||
| Line 229: | Line 218: | ||
!style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|ICD-O-3 Code}} | !style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|ICD-O-3 Code}} | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold"|NET G1 (Grade 1) | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold"|[[NET1|NET]] [[G1]] ([[Grading (tumors)|Grade]] 1) | ||
|All organs | |All [[organs]] | ||
|8240/3 | |8240/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |NET G2 (Grade 2) | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[NET1|NET]] [[G2 phase|G2]] ([[Grading (tumors)|Grade]] 2) | ||
|All organs | |All [[organs]] | ||
|8249/3 | |8249/3 | ||
|- | |- | ||
| rowspan="3" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Neuroendocrine carcinoma | | rowspan="3" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Neuroendocrine]] [[carcinoma]] | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | ||
|All organs | |All [[organs]] | ||
|8246/3 | |8246/3 | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Large cell NEC | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Large cell]] NEC | ||
|All organs | |All [[organs]] | ||
|8013/3 | |8013/3 | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Small cell NEC | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Small cell]] NEC | ||
|All organs | |All [[organs]] | ||
|8041/3 | |8041/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Enterochromaffin cell serotonin-producing NET | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Enterochromaffin cell]] [[serotonin]]-[[Product (biology)|producing]] [[NET1|NET]] | ||
|All organs | |All [[organs]] | ||
|8241/3 | |8241/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Gastrin-producing NET (gastrinoma) | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Gastrin]]-[[Product (biology)|producing]] [[NET1|NET]] ([[gastrinoma]]) | ||
|Stomach, ampulla, small intestine, pancreas | |[[Stomach]], [[ampulla]], [[small intestine]], [[pancreas]] | ||
|8153/3 | |8153/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Glucagon-producing NET (glucagonoma) | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Glucagon]]-[[Product (biology)|producing]] [[NET1|NET]] ([[glucagonoma]]) | ||
|Pancreas | |[[Pancreas]] | ||
|8152/3 | |8152/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Gangliocytic paraganglioma | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Gangliocytic [[paraganglioma]] | ||
|Ampulla, small intestine | |[[Ampulla]], [[small intestine]] | ||
|8683/0 | |8683/0 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Somatostatin-producing NET (somatostatinoma) | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Somatostatin]]-[[Product (biology)|producing]] [[NET1|NET]] (somatostatinoma) | ||
|Ampulla, small intestine, pancreas | |[[Ampulla]], [[small intestine]], [[pancreas]] | ||
|8156/3 | |8156/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Insulin-producing NET (insulinoma) | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Insulin]]-[[Product (biology)|producing]] [[NET1|NET]] ([[insulinoma]]) | ||
|Pancreas | |[[Pancreas]] | ||
|8151/3 | |8151/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |VIPoma | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[VIPoma]] | ||
|Pancreas | |[[Pancreas]] | ||
|8155/3 | |8155/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |L cell, Glucagon-like peptide and PP/PYY-producing NETs | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |L [[Cell (biology)|cell]], [[Glucagon-like peptide-1|Glucagon-like peptide]], and [[PP]]/[[Peptide YY|PYY]]-[[Product (biology)|producing]] [[NET1|NETs]] | ||
|Small intestine, appendix, colorectum | |[[Small intestine]], [[appendix]], colorectum | ||
|8152/1 | |8152/1 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Goblet cell carcinoid | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Goblet cell]] [[carcinoid]] | ||
|Appendix, extrahepatic bile duct | |[[Appendix]], [[Extrahepatic bile ducts|extrahepatic bile duct]] | ||
|8241/3 | |8241/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Tubular carcinoid | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Tubular]] [[carcinoid]] | ||
|Appendix, extrahepatic bile duct | |[[Appendix]], [[Extrahepatic bile ducts|extrahepatic bile duct]] | ||
|8245/1 | |8245/1 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Mixed adenoneuroendocrine carcinoma (MANEC) | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Mixed-handed|Mixed]] adenoneuroendocrine [[carcinoma]] (MANEC) | ||
|All organs | |All [[organs]] | ||
|8244/3 | |8244/3 | ||
|- | |- | ||
| colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Neuroendocrine microadenoma | | colspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Neuroendocrine]] microadenoma | ||
|Pancreas | |[[Pancreas]] | ||
|8150/0 | |8150/0 | ||
|} | |} | ||
===WHO Grading criteria for neuroendocrine neoplasms=== | ===WHO Grading criteria for neuroendocrine neoplasms=== | ||
* WHO grading criteria for neuroendocrine neoplasms is based upon histological markers for cellular proliferation (rather than cellular polymorphism) | *[[WHO]] [[Grading (tumors)|grading]] [[criteria]] for [[neuroendocrine]] [[neoplasms]] is [[Base|based]] upon [[histological]] [[Marker|markers]] for [[cellular]] [[proliferation]] (rather than [[cellular]] [[polymorphism]])<ref name="pmid27259015">{{cite journal| author=Tang LH, Basturk O, Sue JJ, Klimstra DS| title=A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. | journal=Am J Surg Pathol | year= 2016 | volume= 40 | issue= 9 | pages= 1192-202 | pmid=27259015 | doi=10.1097/PAS.0000000000000662 | pmc=4988129 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27259015 }} </ref> | ||
* Following table shows the currently recommended WHO | * Following table shows the currently recommended [[World Health Organization|WHO]] [[Grading (tumors)|grading]] [[criteria]] for all gastroenteropancreatic [[neuroendocrine]] [[neoplasms]]:<ref name="pmid30098710">{{cite journal| author=Inzani F, Petrone G, Rindi G| title=The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. | journal=Endocrinol Metab Clin North Am | year= 2018 | volume= 47 | issue= 3 | pages= 463-470 | pmid=30098710 | doi=10.1016/j.ecl.2018.04.008 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30098710 }} </ref><ref name="pmid22967994">{{cite journal| author=Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P et al.| title=Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. | journal=Ann Oncol | year= 2013 | volume= 24 | issue= 1 | pages= 152-60 | pmid=22967994 | doi=10.1093/annonc/mds276 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22967994 }} </ref><ref name="pmid26113608">{{cite journal| author=Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G et al.| title=Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. | journal=Endocr Relat Cancer | year= 2015 | volume= 22 | issue= 4 | pages= 657-64 | pmid=26113608 | doi=10.1530/ERC-15-0119 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26113608 }} </ref><ref name="pmid25723112">{{cite journal| author=Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM et al.| title=The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. | journal=Am J Surg Pathol | year= 2015 | volume= 39 | issue= 5 | pages= 683-90 | pmid=25723112 | doi=10.1097/PAS.0000000000000408 | pmc=4398606 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25723112 }} </ref><ref name="pmid26482044">{{cite journal| author=Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L et al.| title=Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. | journal=Clin Cancer Res | year= 2016 | volume= 22 | issue= 4 | pages= 1011-7 | pmid=26482044 | doi=10.1158/1078-0432.CCR-15-0548 | pmc=4988130 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26482044 }} </ref><ref name="pmid27169712">{{cite journal| author=La Rosa S, Sessa F, Uccella S| title=Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. | journal=Endocr Pathol | year= 2016 | volume= 27 | issue= 4 | pages= 284-311 | pmid=27169712 | doi=10.1007/s12022-016-9432-9 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27169712 }} </ref><ref name="pmid18360283">{{cite journal| author=Shia J, Tang LH, Weiser MR, Brenner B, Adsay NV, Stelow EB et al.| title=Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? | journal=Am J Surg Pathol | year= 2008 | volume= 32 | issue= 5 | pages= 719-31 | pmid=18360283 | doi=10.1097/PAS.0b013e318159371c | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18360283 }} </ref><ref name="pmid22967994">{{cite journal| author=Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P et al.| title=Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. | journal=Ann Oncol | year= 2013 | volume= 24 | issue= 1 | pages= 152-60 | pmid=22967994 | doi=10.1093/annonc/mds276 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22967994 }} </ref><ref name="pmid26482044">{{cite journal| author=Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L et al.| title=Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. | journal=Clin Cancer Res | year= 2016 | volume= 22 | issue= 4 | pages= 1011-7 | pmid=26482044 | doi=10.1158/1078-0432.CCR-15-0548 | pmc=4988130 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26482044 }} </ref><ref name="pmid24503751">{{cite journal| author=Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM et al.| title=Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. | journal=Am J Surg Pathol | year= 2014 | volume= 38 | issue= 4 | pages= 437-47 | pmid=24503751 | doi=10.1097/PAS.0000000000000169 | pmc=3977000 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24503751 }} </ref> | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 309: | Line 298: | ||
!style="background: #4479BA; width: 150px;" | {{fontcolor|#FFF|Traditional}} | !style="background: #4479BA; width: 150px;" | {{fontcolor|#FFF|Traditional}} | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Neuroendocrine neoplasm GX | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Neuroendocrine]] [[neoplasm]] GX | ||
| colspan="3" |Grade cannot be assessed | | colspan="3" |[[Grading (tumors)|Grade]] cannot be [[Assessment and Plan|assessed]] | ||
|- | |- | ||
| colspan="4" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Well-differentiated GIT NETs & PanNENs: Pancreatic neuroendocrine tumors (PanNETs)''' | | colspan="4" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Well-[[Differentiate|differentiated]] [[GIT]] [[NET1|NETs]] & PanNENs: [[Pancreatic]] [[neuroendocrine]] [[tumors]] (PanNETs)''' | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Neuroendocrine tumor, Grade 1 and PanNET G1 (low grade) | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Neuroendocrine]] [[tumor]], [[Grading (tumors)|Grade]] 1 and PanNET G1 (low [[Grading (tumors)|grade]]) | ||
|<2 | |<2 | ||
|<3 | |<3 | ||
| | | | ||
* Carcinoid tumor | *[[Carcinoid tumor]] | ||
* Islet cell neuroendocrine tumor | *[[Pancreatic islet cell carcinoma|Islet cell neuroendocrine tumor]] | ||
* Pancreatic neuroendocrine tumor | *[[Pancreatic]] [[neuroendocrine]] [[tumor]] | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Neuroendocrine tumor, Grade 2 and PanNET G2 (intermediate grade) | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Neuroendocrine]] [[tumor]], [[Grading (tumors)|Grade]] 2 and PanNET G2 (intermediate [[Grading (tumors)|grade]]) | ||
|2–20 | |2–20 | ||
|3–20 | |3–20 | ||
| | | | ||
* Carcinoid tumor | *[[Carcinoid tumor]] | ||
* Atypical carcinoid tumor (intermediate-grade neuroendocrine tumors of the lung) | * Atypical [[carcinoid tumor]] (intermediate-[[Grading (tumors)|grade]] [[neuroendocrine]] [[tumors]] of the [[lung]]) | ||
* Islet cell neuroendocrine tumor | *[[Pancreatic islet cell carcinoma|Islet cell neuroendocrine tumor]] | ||
* Pancreatic neuroendocrine tumor | *[[Pancreatic]] [[neuroendocrine]] [[tumor]] | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |PanNET G3 | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |PanNET G3 | ||
| Line 336: | Line 325: | ||
|_ | |_ | ||
|- | |- | ||
| colspan="4" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Poorly differentiated PanNENs: Pancreatic neuroendocrine carcinomas (PanNECs)''' | | colspan="4" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Poorly [[Differentiate|differentiated]] PanNENs: [[Pancreatic]] [[neuroendocrine]] [[carcinomas]] (PanNECs)''' | ||
|- | |- | ||
| rowspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Neuroendocrine carcinoma, Grade 3''' and | | rowspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Neuroendocrine]] [[carcinoma]], [[Grading (tumors)|Grade]] 3''' and | ||
'''PanNEC G3 (high grade)''' | '''PanNEC G3 (high [[Grading (tumors)|grade]])''' | ||
| rowspan="2" |>20 | | rowspan="2" |>20 | ||
| rowspan="2" |>20 | | rowspan="2" |>20 | ||
|Small cell carcinoma | |[[Small cell]] [[carcinoma]] | ||
|- | |- | ||
|Large cell neuroendocrine carcinoma | |[[Large cell]] [[neuroendocrine]] [[carcinoma]] | ||
|- | |- | ||
| colspan="4" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN)''' | | colspan="4" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Mixed-handed|Mixed]] [[neuroendocrine]]-non-[[neuroendocrine]] [[neoplasm]] (MiNEN)''' | ||
|} | |} | ||
| Line 356: | Line 345: | ||
!style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|III}} | !style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|III}} | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Incidence, % | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Incidence]], % | ||
|55–88 | |55–88 | ||
|8–13 | |8–13 | ||
| Line 366: | Line 355: | ||
|Single | |Single | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Peritumoral oxyntic mucosa | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Peritumoral [[Oxyntic cells|oxyntic]] [[mucosa]] | ||
|Atrophic | |[[Atrophic]] | ||
|Hypertrophic | |[[Hypertrophic]] | ||
|Normal | |[[Normal]] | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Size, cm | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Size consistency|Size]], [[Centimetre|cm]] | ||
|0.5–1 | |0.5–1 | ||
|<2 | |<2 | ||
|>2 | |>2 | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Location | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Location parameter|Location]] | ||
|Corpus | |Corpus | ||
|Corpus | |Corpus | ||
|Any | |Any | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Sex | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Sex (activity)|Sex]] | ||
|M < F | |[[Male|M]] < [[Female|F]] | ||
|M = F | |[[Male|M]] = [[Female|F]] | ||
|M > F | |[[Male|M]] > [[Female|F]] | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Hypergastrinemia | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Hypergastrinemia | ||
| Line 391: | Line 380: | ||
|No | |No | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Antral G-cell hyperplasia | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Antrum|Antral]] [[G-cells|G-cell]] [[hyperplasia]] | ||
|Yes | |Yes | ||
|No | |No | ||
|No | |No | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Associated disease | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Association (statistics)|Associated]] [[disease]] | ||
| | | | ||
* Chronic atrophic gastritis | *[[Chronic atrophic gastritis]] | ||
| | | | ||
* Multiple endocrine neoplasia 1 | *[[Multiple endocrine neoplasia type 1|Multiple endocrine neoplasia 1]] | ||
* Zollinger–Ellison syndrome | *[[Zollinger-Ellison syndrome|Zollinger–Ellison syndrome]] | ||
|No | |No | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Precursor lesion | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Precursor]] [[lesion]] | ||
|Yes | |Yes | ||
|Yes | |Yes | ||
|No | |No | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |WHO 2010 classification | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[World Health Organization|WHO]] 2010 [[classification]] | ||
|Grade 1 | |[[Grading (tumors)|Grade]] 1 | ||
|Grades 1 or 2 | |[[Grading (tumors)|Grades]] 1 or 2 | ||
|Grades 1–3 | |[[Grading (tumors)|Grades]] 1–3 | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Lymph node metastasis, % | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Lymph node]] [[metastasis]], % | ||
|5 | |5 | ||
|30 | |30 | ||
| Line 426: | Line 415: | ||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|'''WHO 2017'''}} | !style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|'''WHO 2017'''}} | ||
|- | |- | ||
|Islet cell tumor (adenoma/carcinoma) | |[[Pancreatic islet cell carcinoma|Islet cell tumor]] ([[adenoma]]/[[carcinoma]]) | ||
|Well-differentiated endocrine tumor/carcinoma (WDET/WDEC) | |Well-[[Differentiate|differentiated]] [[Endocrine tumors|endocrine tumor]]/[[carcinoma]] (WDET/WDEC) | ||
|NET G1/G2 | |[[NET1|NET]] [[G1]]/[[G2 phase|G2]] | ||
|NET G1/G2/G3 (well-differentiated NEN) | |[[NET1|NET]] [[G1]]/[[G2 phase|G2]]/G3 (well-[[Differentiate|differentiated]] NEN) | ||
|- | |- | ||
|Poorly differentiated endocrine carcinoma | |Poorly [[Differentiate|differentiated]] [[endocrine]] [[carcinoma]] | ||
|Poorly differentiated | |Poorly [[Differentiate|differentiated]] [[endocrine]] [[carcinoma]]/[[small cell]] [[carcinoma]] (PDEC) | ||
|NEC (G3), large cell or small cell type | |NEC (G3), [[large cell]] or [[small cell]] type | ||
|NEC (G3), large cell or small cell type (poorly differentiated NEN) | |NEC (G3), [[large cell]] or [[small cell]] type (poorly [[Differentiate|differentiated]] NEN) | ||
|- | |- | ||
|_ | |_ | ||
|Mixed exocrine-endocrine carcinoma (MEEC) | |[[Mixed-handed|Mixed]] [[exocrine]]-[[endocrine]] [[carcinoma]] (MEEC) | ||
|Mixed adenoneuroendocrine carcinoma | |[[Mixed-handed|Mixed]] adenoneuroendocrine [[carcinoma]] | ||
|Mixed neuroendocrine-non-neuroendocrine neoplasm | |[[Mixed-handed|Mixed]] [[neuroendocrine]]-non-[[neuroendocrine]] [[neoplasm]] | ||
|- | |- | ||
|Pseudotumor lesions | |Pseudotumor [[lesions]] | ||
|Tumor-like lesions (TLLs) | |[[Tumor]]-like [[lesions]] (TLLs) | ||
|Hyperplastic and preneoplastic lesions | |[[Hyperplastic]] and preneoplastic [[lesions]] | ||
|_ | |_ | ||
|} | |} | ||
| Line 450: | Line 439: | ||
===Neuroendocrine system=== | ===Neuroendocrine system=== | ||
*[[Endocrine system]] is a | *[[Endocrine system]] is a signaling [[system]] made of a [[Network effect|network]] of [[glands]] that metabolize hormones. | ||
*[[Neuroendocrine system]] is actually a [[Combination tone|combination]] of | *It secrete hormones in order to lead a series of [[intracellular]] [[cascade]] to get an appropriate response .<ref name="pmid25161467">{{cite journal |vauthors=Oladejo AO |title=GASTROENTEROPANCREATIC NEUROENDOCRINE TUMORS (GEP-NETs) - APPROACH TO DIAGNOSIS AND MANAGEMENT |journal=Ann Ib Postgrad Med |volume=7 |issue=2 |pages=29–33 |date=December 2009 |pmid=25161467 |pmc=4111010 |doi= |url=}}</ref> | ||
* | *[[Neuroendocrine system]] is actually a [[Combination tone|combination]] of both endocrine and nervous system. | ||
*these two [[systems]] have various [[interfaces]] in order to control many functions, specially in [[Gastrointestinal]] system. | |||
*[[Hormones]] | *Gastrointestinal neuroendocrine sustem (GEP-[[NET1|NET]]) is a [[tumor]] of gastrointestinal and neuroendocrine system. | ||
* The vast majority of GEP-[[NET1|NETs]] [[fall]] | * Some of these malignant cells do not form a [[gland]] and are consistent in organ as different cells. (Like G cells in [[stomach]].) | ||
* Despite great | *[[Hormones]] are divided into further sub-types based on their chemical structure: | ||
**[[Peptides]]/[[peptide hormones]] | |||
**[[steroids]] | |||
**[[amine|neuroamines]] | |||
* The vast majority of GEP-[[NET1|NETs]] [[fall]] are further devided in two [[Distinctive feature|distinct]] [[categories]]: | |||
**[[carcinoid]]s | |||
**[[pancreatic]] [[endocrine tumors]] ([[PET|PETs]]). | |||
* Despite great difference in hormone made and the action of these two , they are [[Group (sociology)|grouped]] together as GEP-[[NET1|NETs]] because of [[Similarity matrix|similarities]] in [[cell]] [[Structure factor|structure]].<ref> | |||
"The main two groups of neuroendocrine GEP tumours are so-called carcinoid tumours and endocrine pancreatic tumours" (Öberg 2005a, 90, ). | "The main two groups of neuroendocrine GEP tumours are so-called carcinoid tumours and endocrine pancreatic tumours" (Öberg 2005a, 90, ). | ||
<p> | <p> | ||
| Line 467: | Line 463: | ||
Another way to classify GEP-NETs is to separate those that begin in the glandular neuroendocrine system from those that begin in the diffuse neuroendocrine system. "Neuroendocrine tumors generally may be classified into two categories. The first category is an organ-specific group arising from neuroendocrine organs such as pituitary gland, thyroid, pancreas, and adrenal gland. The second group arises from the diffuse neuroendocrine cells/Kulchitsky cells that are widely distributed throughout the body and are highly concentrated in the pulmonary and gastrointestinal systems" (Liu ''et al.'' 2001, [http://www.nature.com/modpathol/journal/v14/n9/full/3880406a.html]). | Another way to classify GEP-NETs is to separate those that begin in the glandular neuroendocrine system from those that begin in the diffuse neuroendocrine system. "Neuroendocrine tumors generally may be classified into two categories. The first category is an organ-specific group arising from neuroendocrine organs such as pituitary gland, thyroid, pancreas, and adrenal gland. The second group arises from the diffuse neuroendocrine cells/Kulchitsky cells that are widely distributed throughout the body and are highly concentrated in the pulmonary and gastrointestinal systems" (Liu ''et al.'' 2001, [http://www.nature.com/modpathol/journal/v14/n9/full/3880406a.html]). | ||
</ref> | </ref> | ||
*[[Pancreatic]] [[endocrine tumors]] (PETs) are also known as [[endocrine]] [[Pancreatic tumor|pancreatic tumors]] (EPTs) or [[Pancreatic islet cell carcinoma|islet cell tumors]]. | *<nowiki/>[[Pancreatic]] [[endocrine tumors]] (PETs) are also known as [[endocrine]] [[Pancreatic tumor|pancreatic tumors]] (EPTs) or [[Pancreatic islet cell carcinoma|islet cell tumors]]. | ||
* | *further classification of gasterointestinal neuroendocrine tumors is based on: | ||
**[[Secretory component|Secretory]] [[tumors]] are [[Classification|classified]] by the dominant [[hormone]] secreted hormone. | |||
* | **[[Origin (anatomy)|origin]], as [[foregut]] ([[lung]], [[thymus]], [[stomach]], and [[duodenum]]) or [[midgut]] ([[distal]] [[ileum]] and [[proximal]] [[Colon (anatomy)|colon]]) or [[hindgut]] ([[distal]] [[Colon (anatomy)|colon]] and [[rectum]]). | ||
*[[Secretory component|Secretory]] [[tumors]] are [[Classification|classified]] by the [[hormone]] | *[[Carcinoid tumors]] ([[serotonin]] secreting) tend to [[Growth|grow]] much more [[Slow|slowly]] than PETs. | ||
* | |||
* | |||
* In the context of GEP-[[NET1|NETs]], the [[Term logic|terms]] ''[[metastatic]]'' and ''[[malignant]]'' are often [[Usage analysis|used]] interchangeably. | * In the context of GEP-[[NET1|NETs]], the [[Term logic|terms]] ''[[metastatic]]'' and ''[[malignant]]'' are often [[Usage analysis|used]] interchangeably. | ||
| Line 504: | Line 477: | ||
==Causes== | ==Causes== | ||
* In the [[normal]] [[pancreas]], | * In the [[normal]] [[pancreas]], [[Islet cell|islet cells]] [[Product (biology)|produce]] [[hormones]] that [[Regulatory elements|regulate]] a variety of [[Human body|bodily]] [[Function (biology)|functions]], such as the [[control]] of [[blood sugar]] [[Leveling effect|level]] and [[stomach]] [[acid]] [[Product (chemistry)|production]]. | ||
*[[Islet cell]] [[tumors]] include: | *[[Islet cell]] [[tumors]] include: | ||
**[[Gastrinoma|Gastrinomas]] ([[Zollinger-Ellison syndrome]]) | **[[Gastrinoma|Gastrinomas]] ([[Zollinger-Ellison syndrome]]) | ||
**[[Glucagonoma|Glucagonomas]] | **[[Glucagonoma|Glucagonomas]] | ||
**[[Insulinoma|Insulinomas]] | **[[Insulinoma|Insulinomas]] | ||
*The [[Exact test|exact]] [[Causes|cause]] of [[neuroendocrine]] [[tumors]] is [[Cancer of unknown primary origin|unknown]]. | |||
*Different [[DNA mutations]] in [[neuroendocrine cells]] is supposed to be one of the [[causes]] of [[neuroendocrine]] [[tumors]]. | |||
*Various [[hereditary]] [[syndromes]] [[Association (statistics)|associated]] with [[Gastrointestinal tract|GIT]] and pancreatobiliary [[Tract (anatomy)|tract]] [[NET1|NETs]] are mentioned in [[Detailed balance|detail]] in the table below: | *Various [[hereditary]] [[syndromes]] [[Association (statistics)|associated]] with [[Gastrointestinal tract|GIT]] and pancreatobiliary [[Tract (anatomy)|tract]] [[NET1|NETs]] are mentioned in [[Detailed balance|detail]] in the table below: | ||
| Line 521: | Line 496: | ||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Clinical Presentation Outside GIT and Pancreatobiliary Tract}} | !style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Clinical Presentation Outside GIT and Pancreatobiliary Tract}} | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Multiple endocrine neoplasia 1 (MEN 1) | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Multiple endocrine neoplasia type 1|Multiple endocrine neoplasia 1]] ([[MEN 1]]) | ||
| | | | ||
* Autosomal Dominant | *[[Autosomal]] [[Dominant]] | ||
| | | | ||
* 11q13.1 | * 11q13.1 | ||
| | | | ||
* MEN1/ menin | *[[MEN1]]/ menin | ||
| | | | ||
* Nonfunctional | * Nonfunctional | ||
Maybe associated with multiple: | Maybe [[Association (statistics)|associated]] with multiple: | ||
* Gastric NETs (gastrinoma) | *[[Gastric]] [[NET1|NETs]] ([[gastrinoma]]) | ||
* Duodenal NETs | *[[Duodenal]] [[NET1|NETs]] | ||
* Pancreatic NETs (insulinoma) | *[[Pancreatic]] [[NET1|NETs]] ([[insulinoma]]) | ||
| | | | ||
* Esophageal leiomyoma | *[[Esophageal]] [[leiomyoma]] | ||
|More commonly: | |More commonly: | ||
* Pituitary adenoma | *[[Pituitary adenoma]] | ||
* Parathyroid hyperplasia leading to primary hyperparathyroidism | *[[Parathyroid]] [[hyperplasia]] [[Lead|leading]] to [[primary hyperparathyroidism]] | ||
Less commonly: | Less commonly: | ||
* Bronchial NET | *[[Bronchial]] [[NET1|NET]] | ||
* Thymic NET | *[[Thymic]] [[NET1|NET]] | ||
* Adrenal cortical adenoma/tumors | *[[Adrenal]] [[Cortical area|cortical]] [[adenoma]]/[[tumors]] | ||
*Carcinoid tumors | *[[Carcinoid tumors]] | ||
*Nonmedullary thyroid tumors | *Nonmedullary [[Thyroid tumor|thyroid tumors]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |von Hippel-Lindau disease/syndrome (VHL) | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Von Hippel-Lindau disease|von Hippel-Lindau disease/syndrome]] ([[VHL]]) | ||
| | | | ||
* Autosomal Dominant | *[[Autosomal]] [[Dominant]] | ||
| | | | ||
* 3p25.3 | * 3p25.3 | ||
| | | | ||
* VHL/ VHL | *[[VHL]]/ [[VHL]] | ||
| | | | ||
* Nonfunctional or | * Nonfunctional or | ||
*Pancreatic clear cell NETs | *[[Pancreatic]] [[clear cell]] [[NET1|NETs]] | ||
| | | | ||
* Pancreas serous cyst adenomas | *[[Pancreas]] [[serous]] [[cyst]] [[adenomas]] | ||
| | | | ||
* CNS or cerebellar hemangioblastomas | *[[CNS]] or [[cerebellar]] [[Hemangioblastoma|hemangioblastomas]] | ||
* Retinal hemangioblastomas | *[[Retinal]] [[Hemangioblastoma|hemangioblastomas]] | ||
* Renal clear cell carcinomas | *[[Renal]] [[Clear cell tumor|clear cell carcinomas]] | ||
* Pheochromocytomas (often bilateral) | *[[Pheochromocytoma|Pheochromocytomas]] (often [[bilateral]]) | ||
* Adrenal cortical adenomas | *[[Adrenal]] [[Cortical area|cortical]] [[adenomas]] | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Neurofibromatosis 1 | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Neurofibromatosis type I|Neurofibromatosis 1]] | ||
(von Recklinghausen disease) | ([[von Recklinghausen disease]]) | ||
| | | | ||
* Autosomal Dominant | *[[Autosomal]] [[Dominant]] | ||
| | | | ||
* 17q11.2 | * 17q11.2 | ||
| | | | ||
* NF1/ neurofibromin | *[[NF1]]/ [[neurofibromin]] | ||
| | | | ||
* Duodenal somatostatin producing NETs | *[[Duodenal]] [[somatostatin]] [[Product (biology)|producing]] [[NET1|NETs]] | ||
* Pancreatic somatostatin producing NETs | *[[Pancreatic]] [[somatostatin]] [[Product (biology)|producing]] [[NET1|NETs]] | ||
| | | | ||
* Gastrointestinal stromal tumor (GIST) | *[[Gastrointestinal stromal tumor]] ([[GIST]]) | ||
* Neurofibromas | *[[Neurofibroma|Neurofibromas]] | ||
| | | | ||
* Neurofibromatosis | *[[Neurofibromatosis]] | ||
* Cafe´ au lait spots | *[[Café au lait spot|Cafe´ au lait spots]] | ||
* Optic nerve gliomas | *[[Optic nerve gliomas]] | ||
*Pheochromocytoma | *[[Pheochromocytoma]] | ||
|- | |- | ||
| rowspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Tuberous sclerosis | | rowspan="2" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Tuberous sclerosis]] | ||
| rowspan="2" | | | rowspan="2" | | ||
* Autosomal Dominant | *[[Autosomal]] [[Dominant]] | ||
| | | | ||
* 9q34.13 | * 9q34.13 | ||
| | | | ||
* TSC1/ hamartin | *[[TSC1]]/ [[hamartin]] | ||
| rowspan="2" | | | rowspan="2" | | ||
* Pancreatic insulin producing NETs | *[[Pancreatic]] [[insulin]] [[Product (biology)|producing]] [[NET1|NETs]] | ||
* Pancreatic somatostatin producing NETs | *[[Pancreatic]] [[somatostatin]] [[Product (biology)|producing]] [[NET1|NETs]] | ||
| rowspan="2" | | | rowspan="2" | | ||
* Hamartomatous polyp | *[[Hamartomatous]] [[polyp]] | ||
| rowspan="2" | | | rowspan="2" | | ||
* Hamartomas involving multiple organs | *[[Hamartomas]] involving multiple [[organs]] | ||
*Cardiac rhabdomyomas | *[[Cardiac rhabdomyoma|Cardiac rhabdomyomas]] | ||
*Angiomyolipomas | *[[Angiomyolipoma|Angiomyolipomas]] | ||
*Renal cysts | *[[Renal cysts]] | ||
|- | |- | ||
| | | | ||
* 16p13.3 | * 16p13.3 | ||
| | | | ||
* TSC2/ tuberin | *[[TSC2]]/ [[tuberin]] | ||
|} | |} | ||
Other familial syndromes associated with neuroendocrine neoplasms are: | Other [[familial]] [[syndromes]] [[Association (statistics)|associated]] with [[neuroendocrine]] [[neoplasms]] are: | ||
* Multiple Endocrine Neoplasia type 2 (MEN2) | *[[Multiple endocrine neoplasia, type 2|Multiple Endocrine Neoplasia type 2]] ([[MEN2]]) | ||
* Carney complex | *[[Carney complex]] | ||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
===Lung NETs=== | ===Lung NETs=== | ||
* | *The portion of [[lung]] [[neuroendocrine]] [[tumors]] ([[NET1|NETs]]) is approximately 1 to 2% of [[lung]] [[malignancies|malignancy]] in [[Adult|adults]] and 30% of [[neuroendocrine]] tumores.<ref name="pmid28448665">{{cite journal| author=Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y et al.| title=Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. | journal=JAMA Oncol | year= 2017 | volume= 3 | issue= 10 | pages= 1335-1342 | pmid=28448665 | doi=10.1001/jamaoncol.2017.0589 | pmc=5824320 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28448665 }} </ref> | ||
*Annual [[incidence]] of [[lung]] [[NET1|NETs]] was 1.49 per 100,000 [[population]] between 2000 and 2012 according to the [[United States]] Surveillance, [[Epidemiology]], and [[End-group|End]] [[Result|Results]] ([[SEER]]) [[database]]. | |||
*[[Lung]] [[NET1|NETs]] are considered to be the most common primary [[lung neoplasm]] in [[children]] with [[Typical set|typical]] [[Presenting symptom|presentation]] in [[late adolescence]].<ref name="pmid11697843">{{cite journal| author=Quaedvlieg PF, Visser O, Lamers CB, Janssen-Heijen ML, Taal BG| title=Epidemiology and survival in patients with carcinoid disease in The Netherlands. An epidemiological study with 2391 patients. | journal=Ann Oncol | year= 2001 | volume= 12 | issue= 9 | pages= 1295-300 | pmid=11697843 | doi=10.1023/a:1012272314550 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11697843 }} </ref><ref name="pmid12569593">{{cite journal| author=Modlin IM, Lye KD, Kidd M| title=A 5-decade analysis of 13,715 carcinoid tumors. | journal=Cancer | year= 2003 | volume= 97 | issue= 4 | pages= 934-59 | pmid=12569593 | doi=10.1002/cncr.11105 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12569593 }} </ref><ref name="pmid11596039">{{cite journal| author=Hemminki K, Li X| title=Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. | journal=Cancer | year= 2001 | volume= 92 | issue= 8 | pages= 2204-10 | pmid=11596039 | doi=10.1002/1097-0142(20011015)92:8<2204::aid-cncr1564>3.0.co;2-r | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11596039 }} </ref><ref name="pmid18853416">{{cite journal| author=Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK et al.| title=Neuroendocrine tumor epidemiology: contrasting Norway and North America. | journal=Cancer | year= 2008 | volume= 113 | issue= 10 | pages= 2655-64 | pmid=18853416 | doi=10.1002/cncr.23883 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18853416 }} </ref> | |||
* | *[[Average]] [[adult]] [[age]] at the [[Time constant|time]] of [[diagnosis]] of a [[Typical set|typical]] [[lung]] [[NET1|NET]] is 45 [[Year|years]], whereas [[patients]] with atypical [[tumors]] are approximately 10 [[Year|years]] [[Old age|older]].<ref name="pmid12140134">{{cite journal| author=Skuladottir H, Hirsch FR, Hansen HH, Olsen JH| title=Pulmonary neuroendocrine tumors: incidence and prognosis of histological subtypes. A population-based study in Denmark. | journal=Lung Cancer | year= 2002 | volume= 37 | issue= 2 | pages= 127-35 | pmid=12140134 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12140134 }} </ref><ref name="pmid21256263">{{cite journal| author=Cao C, Yan TD, Kennedy C, Hendel N, Bannon PG, McCaughan BC| title=Bronchopulmonary carcinoid tumors: long-term outcomes after resection. | journal=Ann Thorac Surg | year= 2011 | volume= 91 | issue= 2 | pages= 339-43 | pmid=21256263 | doi=10.1016/j.athoracsur.2010.08.062 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21256263 }} </ref> | ||
* | *[[Incidence rate|Incidence]] of [[lung]] [[NET1|NETs]] differs from 0.2 to 2 per 100,000 [[population]] per [[year]] . | ||
* | *There's a [[Higher Power|higher]] [[incidence]] of [[lung]] [[NET1|NETs]] in [[Womens Pack|women]] as [[Comparability|compared]] to [[men]]. | ||
*Lung NET has annual [[Incidence rate|incidence rates]] of 0.2 and 1.3 per 100,000 [[population]] among [[men]] and [[Womens Pack|women]] respectively. | |||
*[[White (mutation)|White]] [[People's Solidarity|people]] have [[Higher Power|higher]] [[incidence]] of [[lung]] [[NET1|NETs]] as [[Comparability|compared]] to [[black]] [[People's Solidarity|people]].<ref name="pmid11399686">{{cite journal| author=Fink G, Krelbaum T, Yellin A, Bendayan D, Saute M, Glazer M et al.| title=Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. | journal=Chest | year= 2001 | volume= 119 | issue= 6 | pages= 1647-51 | pmid=11399686 | doi=10.1378/chest.119.6.1647 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11399686 }} </ref><ref name="pmid16455477">{{cite journal| author=Gatta G, Ciccolallo L, Kunkler I, Capocaccia R, Berrino F, Coleman MP et al.| title=Survival from rare cancer in adults: a population-based study. | journal=Lancet Oncol | year= 2006 | volume= 7 | issue= 2 | pages= 132-40 | pmid=16455477 | doi=10.1016/S1470-2045(05)70471-X | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16455477 }} </ref><ref name="pmid28448665">{{cite journal| author=Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y et al.| title=Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. | journal=JAMA Oncol | year= 2017 | volume= 3 | issue= 10 | pages= 1335-1342 | pmid=28448665 | doi=10.1001/jamaoncol.2017.0589 | pmc=5824320 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28448665 }} </ref> | |||
===Digestive system NETs=== | ===Digestive system NETs=== | ||
* | *Annual [[incidence]] of [[digestive system]] [[NET1|NETs]] is 3.56 per 100,000 in United States approximately. | ||
*Pancreatic | *[[Digestive system]] [[NET1|NENs]] of the [[tubular]] [[gastrointestinal tract]] and [[pancreas]] are [[Relatively compact|relatively]] rare with an [[incidence]] of ≤1 case per 100,000 [[Individual growth|individuals]] per [[year]]..<ref name="pmid28448665">{{cite journal| author=Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y et al.| title=Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. | journal=JAMA Oncol | year= 2017 | volume= 3 | issue= 10 | pages= 1335-1342 | pmid=28448665 | doi=10.1001/jamaoncol.2017.0589 | pmc=5824320 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28448665 }} </ref> | ||
*[[Pancreatic]] [[NET1|NETs]] account for 1 to 2% (<3%) of all primary [[Pancreatic neoplasm|pancreatic neoplasms]].<ref name="pmid20058030">{{cite journal| author=Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W et al.| title=Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. | journal=J Gastroenterol | year= 2010 | volume= 45 | issue= 2 | pages= 234-43 | pmid=20058030 | doi=10.1007/s00535-009-0194-8 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20058030 }} </ref><ref name="pmid24499825">{{cite journal| author=Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K et al.| title=Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. | journal=J Gastroenterol | year= 2015 | volume= 50 | issue= 1 | pages= 58-64 | pmid=24499825 | doi=10.1007/s00535-014-0934-2 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24499825 }} </ref><ref name="pmid18536315">{{cite journal| author=Ito T, Tanaka M, Imamura M, Neuroendocrine Tumor Workshop Japan| title=[Results of a nationwide survey of gastrointestinal tumors in Japan]. | journal=Nihon Geka Gakkai Zasshi | year= 2008 | volume= 109 | issue= 3 | pages= 128-32 | pmid=18536315 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18536315 }} </ref><ref name="pmid17671766">{{cite journal| author=Ito T, Tanaka M, Sasano H, Osamura YR, Sasaki I, Kimura W et al.| title=Preliminary results of a Japanese nationwide survey of neuroendocrine gastrointestinal tumors. | journal=J Gastroenterol | year= 2007 | volume= 42 | issue= 6 | pages= 497-500 | pmid=17671766 | doi=10.1007/s00535-007-2056-6 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17671766 }} </ref> | |||
*Following is a table that shows [[Association (statistics)|association]] of different [[diseases]] with the [[incidence]] of [[pancreatic]] [[NET1|NETs]]: | |||
{| class="wikitable" | |||
|+ | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Disease/Syndrome}} | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|% chance of developing Pancreatic NET}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Multiple endocrine neoplasia type 1|Multiple endocrine neoplasia 1]] '''('''[[MEN 1]]''')''' | |||
|80-100% | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Von Hippel-Lindau disease|von Hippel-Lindau disease/syndrome]] '''('''[[VHL]]''')''' | |||
|20% | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Neurofibromatosis type I|Neurofibromatosis 1]] | |||
([[von Recklinghausen disease]]) | |||
|10% | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Tuberous sclerosis]] | |||
|1% | |||
|} | |||
==Risk factors== | ==Risk factors== | ||
*Smoking is a risk factor for both lung and pancreatic NETs especially in case of atypical tumors.<ref name="pmid11399686">{{cite journal| author=Fink G, Krelbaum T, Yellin A, Bendayan D, Saute M, Glazer M et al.| title=Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. | journal=Chest | year= 2001 | volume= 119 | issue= 6 | pages= 1647-51 | pmid=11399686 | doi=10.1378/chest.119.6.1647 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11399686 }} </ref><ref name="pmid8604242">{{cite journal| author=Froudarakis M, Fournel P, Burgard G, Bouros D, Boucheron S, Siafakas NM et al.| title=Bronchial carcinoids. A review of 22 cases. | journal=Oncology | year= 1996 | volume= 53 | issue= 2 | pages= 153-8 | pmid=8604242 | doi=10.1159/000227552 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8604242 }} </ref><ref name="pmid18491401">{{cite journal| author=Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC| title=Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. | journal=Int J Cancer | year= 2008 | volume= 123 | issue= 4 | pages= 867-73 | pmid=18491401 | doi=10.1002/ijc.23529 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18491401 }} </ref> | [[Fewmets|Few]] of the [[risk factors]] for [[neuroendocrine]] [[tumors]] include: | ||
*Inherited predisposition | |||
*Diabetes is associated with increased risk of pancreatic NETs | *[[Smoking]] is a [[risk factor]] for both [[lung]] and [[pancreatic]] [[NET1|NETs]] especially in case of atypical [[tumors]].<ref name="pmid11399686">{{cite journal| author=Fink G, Krelbaum T, Yellin A, Bendayan D, Saute M, Glazer M et al.| title=Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. | journal=Chest | year= 2001 | volume= 119 | issue= 6 | pages= 1647-51 | pmid=11399686 | doi=10.1378/chest.119.6.1647 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11399686 }} </ref><ref name="pmid8604242">{{cite journal| author=Froudarakis M, Fournel P, Burgard G, Bouros D, Boucheron S, Siafakas NM et al.| title=Bronchial carcinoids. A review of 22 cases. | journal=Oncology | year= 1996 | volume= 53 | issue= 2 | pages= 153-8 | pmid=8604242 | doi=10.1159/000227552 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8604242 }} </ref><ref name="pmid18491401">{{cite journal| author=Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC| title=Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. | journal=Int J Cancer | year= 2008 | volume= 123 | issue= 4 | pages= 867-73 | pmid=18491401 | doi=10.1002/ijc.23529 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18491401 }} </ref> | ||
*Chronic pancreatitis is associated with increased risk of pancreatic NETs | *[[Genetic predisposition|Inherited predisposition]] is one of the [[risk factors]] especially in the case of [[lung]] [[NET1|NETs]] (not [[Related phenomena|related]] to [[MEN syndromes|MEN syndrome]]).<ref name="pmid11391591">{{cite journal| author=Oliveira AM, Tazelaar HD, Wentzlaff KA, Kosugi NS, Hai N, Benson A et al.| title=Familial pulmonary carcinoid tumors. | journal=Cancer | year= 2001 | volume= 91 | issue= 11 | pages= 2104-9 | pmid=11391591 | doi=10.1002/1097-0142(20010601)91:11<2104::aid-cncr1238>3.0.co;2-i | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11391591 }} </ref> | ||
*[[Diabetes]] is [[Association (statistics)|associated]] with increased [[RiskMetrics|risk]] of [[pancreatic]] [[NET1|NETs]]. | |||
*[[Chronic pancreatitis]] is [[Association (statistics)|associated]] with increased [[RiskMetrics|risk]] of [[pancreatic]] [[NET1|NETs]]. | |||
*The [[inherited]] [[Genetic Disorders|genetic]] [[syndromes]] [[Association (statistics)|associated]] with increased [[Risk factor|risk]] of [[Causes|causing]] [[neuroendocrine]] [[tumors]] include: | |||
**[[Multiple endocrine neoplasia type 1|Multiple Endocrine Neoplasia type 1]] ([[MEN1]]) | |||
**[[Multiple endocrine neoplasia type 2|Multiple Endocrine Neoplasia type 2]] ([[MEN2]]) | |||
**[[Von Hippel-Lindau syndrome]] | |||
**[[Neurofibromatosis]] | |||
**[[Tuberous sclerosis]] | |||
==Natural History, Complications and Prognosis== | ==Natural History, Complications and Prognosis== | ||
* Diabetes | |||
* Hormone | * [[Neuroendocrine]] [[tumors]] are [[slow]] [[Growth|growing]] [[tumors]] but they can [[Product (biology)|produce]] [[amino acids]] that can [[Causes|cause]] severe [[symptoms]]. | ||
* Severe low blood sugar (from insulinomas) | |||
* Severe ulcers in the stomach and small intestine (from gastrinomas) | ===Complications=== | ||
* Spread of the tumor to the liver | *Common [[complications]] of [[neuroendocrine]] [[tumors]] include the following: | ||
**[[Diabetes]] | |||
**[[Hormone]] [[Crisis (charity)|crisis]] (if the [[tumor]] [[Release (information centre)|releases]] certain types of [[hormones]]) | |||
** Severe [[low blood sugar]] (from [[Insulinoma|insulinomas]]) | |||
** Severe [[ulcers]] in the [[stomach]] and [[small intestine]] (from [[Gastrinoma|gastrinomas]]) | |||
**[[Spread of the cancer|Spread of the tumor]] to the [[liver]] | |||
===Prognosis=== | ===Prognosis=== | ||
* | *[[Neuroendocrine]] [[tumors]] can be [[Cure for cancer|cured]] if they are removed [[Surgery operation|surgically]] before their [[Spread of the cancer|spread]] to the other [[organs]]. | ||
* Life-threatening problems (such as very low blood sugar) can occur due to excess hormone production, or if the cancer spreads throughout the body. | *[[Chemotherapy]] can be [[Usage analysis|used]] in case of [[cancerous]] [[tumors]] but it usually doesn't [[cure]] the [[patients]]. | ||
* | *[[Life]]-threatening [[Problem Solved|problems]] (such as a very [[Low blood sugar|low blood sugar level]]) can occur due to [[Excess risk|excess]] [[hormone]] [[Product (biology)|production]], or if [[Spread of the cancer|cancer spreads]] throughout the [[Human body|body]]. | ||
*[[Fewmets|Few]] of the [[prognostic]] [[Fact table|facts]] about poorly [[Differentiate|differentiated]] [[pancreatic]] NEC are given below:<ref name="pmid24503751" /> | |||
**88% of the [[patients]] have [[lymph node]] or [[Distance matrix|distant]] [[metastatic disease]] at the [[Time constant|time]] of [[tumor]] [[Presenting symptom|presentation]]. | |||
**7% of the [[patients]] subsequently [[Development (biology)|develop]] [[metastases]]. | |||
**The [[median]] [[Survival analysis|survival]] is 11 months, [[Range (statistics)|ranging]] from 0 to 104 months. | |||
**The two-[[year]] [[survival rate]] is 22.5%. | |||
**The [[five-year survival rate]] is 16.1%. | |||
==History and Symptoms== | ==History and Symptoms== | ||
* | |||
* | * Initially, many of the [[neuroendocrine]] [[tumors]] mostly remain [[asymptomatic]]. | ||
**[[Flushing]] | *When they [[Causes|cause]] [[symptoms]], it is usually due to the following two [[Reasoning|reasons]]: | ||
**[[Diarrhea]] or increase in number of bowel movements | **[[Tumor]] [[Location parameter|location]] [[Causality|causing]]: | ||
***[[Feeling]] of a [[Growth|growing]] [[lump]] under the [[skin]] | |||
***Persistent [[pain]] occurring from increasing [[tumor]] [[Size consistency|size]] during its [[growth]] in [[Specific activity|specific]] [[area]] | |||
***Unusually [[tired feeling]] ([[fatigue]]) | |||
***[[Unintentional weight loss]] | |||
***[[Loss of appetite]] | |||
***[[Headache]] | |||
***[[Jaundice]] | |||
***[[Changes in bowel habits|Changes in bowel]] or [[Urinary bladder|bladder]] habits | |||
***Unusual [[discharge]] or [[bleeding]] | |||
***[[Persistent cough]] or [[hoarseness]] | |||
***Persistent [[fever]] or [[night sweats]] | |||
**Particular [[Excess risk|excessive]] [[hormone]] [[Product (biology)|production]] by the [[tumor]] ([[Function (biology)|functional]] [[tumor]]) | |||
===Carcinoid tumor=== | |||
*[[Carcinoid tumors]] may [[Product (biology)|produce]] [[serotonin]] ([[5-HT]]), a [[biogenic amine]] [[Lead|leading]] to the following [[Specific activity|specific]] [[set]] of [[symptoms]] which are collectively known as [[Carcinoid syndrome]]: | |||
**[[Unusual warmth and flushing of skin|Flushing of skin]] | |||
**[[Diarrhea]] (or increase in the [[number]] of [[bowel]] movements) | |||
**[[Weight loss]] | **[[Weight loss]] | ||
**[[Weight gain]] | **[[Weight gain]] | ||
| Line 661: | Line 694: | ||
**[[Acromegaly]] | **[[Acromegaly]] | ||
**[[Cushing's syndrome]] | **[[Cushing's syndrome]] | ||
* | ===Pancreatic NETs=== | ||
*According to the [[Recent changes|recent]] [[Study design|studies]], 50 to 85% of the [[pancreatic]] [[NET1|NETs]] are nonfunctioning. | |||
*Functioning pancreatic NETs | *Functioning [[pancreatic]] [[NET1|NETs]] include the following: | ||
**Insulinomas | **'''[[Insulinoma|Insulinomas]]''' with [[Typical set|typical]] [[Presenting symptom|presentation]] of episodic [[hypoglycemia]] [[Lead|leading]] to: | ||
**Gastrinomas | ***[[Confusion]] | ||
** | ***[[Amnesia]] (during [[hypoglycemic]] [[Phase (waves)|phase]] is common) | ||
** | ***[[Visual]] [[Change detection|changes]] | ||
***[[Palpitations]] | |||
***[[Trembling|Tremulousness]] | |||
***[[Diaphoresis]] | |||
***Unusual [[behavior]] | |||
**'''[[Gastrinoma|Gastrinomas]]''' with [[Typical set|typical]] [[Presenting symptoms|presentation]] of: | |||
***[[Peptic ulcer disease]] | |||
***[[Diarrhea]] (prominent [[Features (pattern recognition)|feature]] too sometimes) | |||
**'''[[Glucagonoma]]''' [[Causes|causing]] classical [[clinical]] [[syndrome]] which includes: | |||
***[[Necrolytic migratory erythema]] | |||
***[[Cheilitis]] | |||
***[[Diabetes mellitus]] | |||
***[[Anemia]] | |||
***[[Weight loss]] | |||
***[[Diarrhea]] | |||
***[[Venous thrombosis]] | |||
***[[Neuropsychiatric]] [[symptoms]] | |||
**'''[[VIPoma]]''' [[syndrome]] [[Presenting symptom|presents]] with the following main [[clinical]] [[symptoms]]: | |||
***[[Watery diarrhea]] | |||
***[[Hypokalemia]] | |||
***[[Hypochlorhydria]] | |||
==Laboratory Findings== | ==Laboratory Findings== | ||
* | * Many [[neuroendocrine]] [[Tumor cell|tumor cells]] especially possess [[strong]] [[receptors]] on their [[Surface chemistry|surfaces]] through which they receive [[hormonal]] [[Message-passing method|messages]] such as: | ||
**[[PET|PETs]] often have [[strong]] [[receptors]] for [[somatostatin]]. | |||
**Such [[Tumor cell|tumor cells]] ''[[overexpress]]'' the [[somatostatin receptors]] (SSTRs) and are thus considered to be ''[[Avidity|avid]] for'' this [[hormone]], hence, their ''[[Uptake signal sequence|uptake]]'' of [[somatostatin]] is [[strong]]. | |||
**This [[avidity]] for [[somatostatin]] is the key factor for the [[PET|PETs']] [[diagnosis]] and it also [[MakeBot|makes]] the [[tumors]] [[Vulnerable populations|vulnerable]] to [[Certain safety factor|certain]] ''[[Targeted therapy|targeted therapies]]''. | |||
* | *[[Generality|Generally]], the important common [[Usage analysis|uses]] of [[tumor markers]] include: | ||
**[[Help Menu|Helpful]] in the [[Specific activity|specific]] [[tumor]] [[diagnosis]]. | |||
**[[Help Menu|Helpful]] in [[Tracking changes|tracking]] the progress of [[tumor]] [[therapy]] hence, the detrimental [[side effects]] of [[Contrast agent|contrast]] [[CT scan]] on the [[patient]] can be [[Avoidance reaction|avoided]]. | |||
{| class="wikitable" | {| class="wikitable" | ||
| Line 684: | Line 742: | ||
|- | |- | ||
| | | | ||
*[[Chromogranin]] | *[[Chromogranin A]] (CgA) | ||
* Urine 5-hydroxy indole acetic acid (5-HIAA) (grade C) | *[[Urine]] [[5-Hydroxyindoleacetic acid|5-hydroxy indole acetic acid]] ([[5-HIAA]]) ([[Grading (tumors)|grade]] C) | ||
* Neuron-specific enolase (NSE, gamma-gamma dimer) | *[[Neuron-specific enolase]] (NSE, [[gamma]]-[[gamma]] [[dimer]]) | ||
* Synaptophysin (P38) | *[[Synaptophysin]] ([[P38]]) | ||
| | | | ||
*Synaptobrevin (VAMP-1) | *[[Synaptobrevin]] ([[VAMP1|VAMP-1]]) | ||
* Synapsin (1A, 1B, 2A, 2B) | *[[Synapsin]] ([[Synapsin I|1A]], [[Synapsin I|1B]], [[Synapsin 2|2A]], [[Synapsin 2|2B]]) | ||
* SV2 | *[[SV2A|SV2]] | ||
* Protein P65 | *[[Protein]] P65 | ||
* Protein S-100 | *[[S-100 protein|Protein S-100]] | ||
* Protein gene product (PGP) 9.5 | *[[Protein]] [[gene product]] ([[PGPEP1|PGP]]) 9.5 | ||
* Intermediate filaments (cytokeratins, vimentin, neurofilaments) | *[[Intermediate filaments]] ([[Cytokeratin|cytokeratins]], [[vimentin]], [[Neurofilament|neurofilaments]]) | ||
* Protein 7B2 | *[[Protein]] 7B2 | ||
* Chromogranin B (secretogranin I) | *[[Chromogranin]] B (secretogranin I) | ||
* Chromogranin C (secretogranin II) | *[[Chromogranin]] C (secretogranin II) | ||
* Pancreastatin | *[[Pancreastatin]] | ||
* Vasostatin | * Vasostatin | ||
* Cytochrome b561 | *[[Cytochrome]] b561 | ||
* Leu-7 (HNK-1) | * Leu-7 (HNK-1) | ||
* Calcitonin | *[[Calcitonin]] | ||
* Human chorionic gonadotropin-alpha (HCG-α) | *[[Human chorionic gonadotropin]]-alpha ([[HCG]]-α) | ||
* Human chorionic gonadotropin-beta (HCG-β) | *[[Human chorionic gonadotropin]]-beta ([[HCG]]-β) | ||
* Thyroid function tests (TFTs) | *[[Thyroid function tests]] ([[TFTs]]) | ||
* Parathyroid hormone (PTH) | *[[Parathyroid hormone]] ([[PTH]]) | ||
* Calcium | *[[Calcium]] | ||
| | | | ||
*Prolactin | *[[Prolactin]] | ||
* | *[[Alpha-fetoprotein]] | ||
* Carcinoembryonic antigen (CEA) | *[[Carcinoembryonic antigen]] ([[CEA]]) | ||
*ß-human chorionic gonadotrophin (ß-HCG) (grade D) | *ß-[[human chorionic gonadotrophin]] (ß-[[HCG]]) ([[Grading (tumors)|grade]] D) | ||
* CGRP | *[[CGRP]] | ||
* GRP | * GRP | ||
* PYY | *[[Peptide YY|PYY]] | ||
* hCGα | *[[HCG|hCGα]] | ||
* N Peptide K | * N [[Peptide]] K | ||
* Neurokinin A | *[[Neurokinin A]] | ||
* Serotonin | *[[Serotonin]] | ||
* Neurotensin | *[[Neurotensin]] | ||
* Motilin | *[[Motilin]] | ||
* Substance P | *[[Substance P]] | ||
* Histamine | *[[Histamine]] | ||
* Catecholamines | *[[Catecholamines]] | ||
* Dopa | *[[Dopamine|Dopa]] | ||
* Various rarer peptide hormones | * Various [[Rare|rarer]] [[peptide hormones]] | ||
* Synaptotagmin | *[[Synaptotagmin]] | ||
* HISL-19 | * HISL-19 | ||
| | | | ||
*N-terminally truncated variant of heat shock protein 70 (Hsp70) | *N-terminally [[Truncated distribution|truncated]] variant of [[Heat shock protein 70 (Hsp70) internal ribosome entry site (IRES)|heat shock protein 70]] ([[Hsp70]]) | ||
* CDX-2, a homeobox gene product | *[[CDX2|CDX-2]], a [[homeobox gene]] [[Product (biology)|product]] | ||
* Neuroendocrine secretory protein-55 | *[[Neuroendocrine]] [[secretory protein]]-55 | ||
|} | |} | ||
==CT Scan== | ==CT Scan== | ||
* | *CT scan is one of the most common diagnostic tool used for diagnosing of Neuroendocrine tumors. | ||
*CT | *CT scan with contrast medium can detect 95% of the tumors with size of >3 cm in size, and no tumors under 1 cm (University of Michigan Medical School n. d., [http://www.med.umich.edu/lrc/presentation/endo/islet.htm]). | ||
==PET Scan== | ==PET Scan== | ||
| Line 771: | Line 829: | ||
Two tricky issues in evaluating therapies are durability (is the therapy long-lasting?) and stasis (are the tumors neither growing nor shrinking?). For example, one therapy might give good initial results – but within months the benefit evaporates. And another therapy might be disparaged by some for causing very little tumor shrinkage, but be championed by others for causing significant tumoristasis. | Two tricky issues in evaluating therapies are durability (is the therapy long-lasting?) and stasis (are the tumors neither growing nor shrinking?). For example, one therapy might give good initial results – but within months the benefit evaporates. And another therapy might be disparaged by some for causing very little tumor shrinkage, but be championed by others for causing significant tumoristasis. | ||
The half-life of somatostatin in circulation is under three minutes, making it useless for diagnosis and targeted therapies. For this reason, The synthetic forms are typically called ''somatostatin analogs'' (''somatostatin analogues''), but according to the US [[Food and Drug Administration]] (FDA), the proper term is | The half-life of somatostatin in circulation is under three minutes, making it useless for diagnosis and targeted therapies. For this reason, The synthetic forms are typically called ''somatostatin analogs'' (''somatostatin analogues''), but according to the US [[Food and Drug Administration]] (FDA), the proper term is somatostatin congeners. (In this article we conform to the old terminology, as the medical community has been slow to adopt the term ''[[congener]]''.) The analogs have a much longer half-life than somatostatin, and other properties that make them more suitable for diagnosis and therapy. | ||
===Chemotherapy=== | ===Chemotherapy=== | ||
| Line 828: | Line 886: | ||
</ref> | </ref> | ||
====Other | ====Other Therapies==== | ||
* '''Radiofrequency ablation''' (RFA) is used when a patient has relatively few metastases. In RFA, a needle is inserted into the center of the lesion and is vibrated at high frequency to generate heat; the tumor cells are killed by cooking. | * '''Radiofrequency ablation''' (RFA) is used when a patient has relatively few metastases. In RFA, a needle is inserted into the center of the lesion and is vibrated at high frequency to generate heat; the tumor cells are killed by cooking. | ||
Latest revision as of 18:45, 22 September 2020
|
Neuroendocrine tumors Microchapters |
For patient information, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [9] Associate Editor(s)-in-Chief: Sara Mohsin, M.D.[10]
Overview
Neuroendocrine tumors, or more properly gastro-entero-pancreatic or gastroenteropancreatic neuroendocrine tumors (GEP-NETs), are cancers of the interface between the endocrine (hormonal) system and the nervous system.
Historical Perspective
- In 1907, Siegfried Oberndorfer was the first person to clearly distinguish GEP-NETs from other forms of cancer. Since they were so slow-growing, he considered them to be "cancer-like" rather than truly cancerous, and hence, he coined the term "carcinoid" for these tumors.
- In 1929, Siegfried Oberndorfer reported that some such tumors were not so indolent and distinguished them as PETs (mostly called carcinoids). Despite of the differences between the two categories, even in the twenty-first century, some doctors (including oncologists) insist on calling all GEP-NETs "carcinoid".
- In 1988, the earliest synthetic form of somatostatin used in the treatment of neuroendocrine tumors was octreotide, which was first marketed by Sandoz as Sandostatin.
- In 2006 and 2007, the European Neuroendocrine Tumor Society (ENETS) proposed a staging scheme same as for most other types of epithelial neoplasms for GEP NENs, alongwith a histologic grading system applicable to all disease stages. American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) later on endorsed this grading proposal for the tumor, node, metastasis (TNM) staging classification of digestive system NENs, after modifying the staging parameters of the ENETS proposal.
- Th 2010 WHO classification of tumors of the gastrointestinal tract, liver, and pancreas (which was subsequently updated in 2017) also endorsed the ENETS grading scheme for NENs of the digestive tract, separating well-differentiated tumors into low-grade (G1) and intermediate-grade (G2) categories. All poorly differentiated NETs are high-grade (G3) NECs according to this classification scheme.
Classification
Human GEP-NETs by Site of Origin and by Symptom
Simplified classification according to anatomic distribution
Classification of GEP-NETs by cell characteristics
- The diverse and amorphous nature of GEP-NETs has led to a confused, overlapping, and changing terminology.
- In general, aggressiveness (malignancy), secretion (of hormones), and anaplasia (dissimilarity between tumor cells and normal cells) tend to go together, but there are many exceptions, which have contributed to the confusion in terminology. For example, the term atypical carcinoid is sometimes used to indicate an aggressive tumor without secretions, whether anaplastic or well-differentiated.
- In 2000, the World Health Organization (WHO) revised the classification of GEP-NETs, abandoning the term carcinoid in favor of neuroendocrine tumor (NET) and abandoning islet cell tumor or pancreatic endocrine tumor for neuroendocrine carcinoma (NEC).
- Judging from papers published into 2006, the medical community is accepting this new terminology with great sluggishness. (Perhaps one reason for the resistance is that the WHO chose to label the least aggressive subclass of neuroendocrine neoplasm with the term – neuroendocrine tumor – widely used previously either for the superclass or for the generally aggressive noncarcinoid subclass).
- Klöppel et alia have written an overview that clarifies the WHO classification and bridges the gap to the old terminology (Klöppel, Perren, and Heitz 2004), this old terminology is given in the table below:
Summary of classification by cell characteristics (the WHO classification)
- This classification was done due to peripheral receptors.
- GEP-NETs receptors (APUDomas) are the main detected receptors.
- That term is now misleading, since it is based on a discredited theory of the development of the tumors.
- It is now known that this receptor may not be indicator of cell origin.[1]
| Superclass:Öberg, WHO, Klöppel et alia: Gastro-entero-pancreatic neuroendocrine tumor (GEP-NET) | ||||
|---|---|---|---|---|
| Subclass 1 (less malignant) | Subclass 2 (more malignant) | Subclass 3 (most malignant) | Subclass 4 (mixed) | Subclass 5 (miscellaneous) |
|
|
|
| |
2010 WHO Classification Of NET
- 2010 WHO Classification of neuroendocrine tumors with their ICD-O-3 codes is given below:
| NET (Neuroendocrine Tumor) Classification | Location | ICD-O-3 Code | |
|---|---|---|---|
| NET G1 (Grade 1) | All organs | 8240/3 | |
| NET G2 (Grade 2) | All organs | 8249/3 | |
| Neuroendocrine carcinoma | All organs | 8246/3 | |
| Large cell NEC | All organs | 8013/3 | |
| Small cell NEC | All organs | 8041/3 | |
| Enterochromaffin cell serotonin-producing NET | All organs | 8241/3 | |
| Gastrin-producing NET (gastrinoma) | Stomach, ampulla, small intestine, pancreas | 8153/3 | |
| Glucagon-producing NET (glucagonoma) | Pancreas | 8152/3 | |
| Gangliocytic paraganglioma | Ampulla, small intestine | 8683/0 | |
| Somatostatin-producing NET (somatostatinoma) | Ampulla, small intestine, pancreas | 8156/3 | |
| Insulin-producing NET (insulinoma) | Pancreas | 8151/3 | |
| VIPoma | Pancreas | 8155/3 | |
| L cell, Glucagon-like peptide, and PP/PYY-producing NETs | Small intestine, appendix, colorectum | 8152/1 | |
| Goblet cell carcinoid | Appendix, extrahepatic bile duct | 8241/3 | |
| Tubular carcinoid | Appendix, extrahepatic bile duct | 8245/1 | |
| Mixed adenoneuroendocrine carcinoma (MANEC) | All organs | 8244/3 | |
| Neuroendocrine microadenoma | Pancreas | 8150/0 | |
WHO Grading criteria for neuroendocrine neoplasms
- WHO grading criteria for neuroendocrine neoplasms is based upon histological markers for cellular proliferation (rather than cellular polymorphism)[2]
- Following table shows the currently recommended WHO grading criteria for all gastroenteropancreatic neuroendocrine neoplasms:[3][4][5][6][7][8][9][4][7][10]
| Grade/Classification | Mitotic Count/ 10 HPFs (High-Power Field) | Ki-67 Labeling Index, % | Traditional |
|---|---|---|---|
| Neuroendocrine neoplasm GX | Grade cannot be assessed | ||
| Well-differentiated GIT NETs & PanNENs: Pancreatic neuroendocrine tumors (PanNETs) | |||
| Neuroendocrine tumor, Grade 1 and PanNET G1 (low grade) | <2 | <3 | |
| Neuroendocrine tumor, Grade 2 and PanNET G2 (intermediate grade) | 2–20 | 3–20 |
|
| PanNET G3 | >20 | >20 | _ |
| Poorly differentiated PanNENs: Pancreatic neuroendocrine carcinomas (PanNECs) | |||
| Neuroendocrine carcinoma, Grade 3 and
PanNEC G3 (high grade) |
>20 | >20 | Small cell carcinoma |
| Large cell neuroendocrine carcinoma | |||
| Mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) | |||
| Classification | I | II | III |
|---|---|---|---|
| Incidence, % | 55–88 | 8–13 | 12–23 |
| Multifocality | Multiple | Multiple | Single |
| Peritumoral oxyntic mucosa | Atrophic | Hypertrophic | Normal |
| Size, cm | 0.5–1 | <2 | >2 |
| Location | Corpus | Corpus | Any |
| Sex | M < F | M = F | M > F |
| Hypergastrinemia | Yes | Yes | No |
| Antral G-cell hyperplasia | Yes | No | No |
| Associated disease | No | ||
| Precursor lesion | Yes | Yes | No |
| WHO 2010 classification | Grade 1 | Grades 1 or 2 | Grades 1–3 |
| Lymph node metastasis, % | 5 | 30 | 70 |
| WHO 1980 | WHO 2000/2004 | WHO 2010 | WHO 2017 |
|---|---|---|---|
| Islet cell tumor (adenoma/carcinoma) | Well-differentiated endocrine tumor/carcinoma (WDET/WDEC) | NET G1/G2 | NET G1/G2/G3 (well-differentiated NEN) |
| Poorly differentiated endocrine carcinoma | Poorly differentiated endocrine carcinoma/small cell carcinoma (PDEC) | NEC (G3), large cell or small cell type | NEC (G3), large cell or small cell type (poorly differentiated NEN) |
| _ | Mixed exocrine-endocrine carcinoma (MEEC) | Mixed adenoneuroendocrine carcinoma | Mixed neuroendocrine-non-neuroendocrine neoplasm |
| Pseudotumor lesions | Tumor-like lesions (TLLs) | Hyperplastic and preneoplastic lesions | _ |
Pathophysiology
Neuroendocrine system
- Endocrine system is a signaling system made of a network of glands that metabolize hormones.
- It secrete hormones in order to lead a series of intracellular cascade to get an appropriate response .[11]
- Neuroendocrine system is actually a combination of both endocrine and nervous system.
- these two systems have various interfaces in order to control many functions, specially in Gastrointestinal system.
- Gastrointestinal neuroendocrine sustem (GEP-NET) is a tumor of gastrointestinal and neuroendocrine system.
- Some of these malignant cells do not form a gland and are consistent in organ as different cells. (Like G cells in stomach.)
- Hormones are divided into further sub-types based on their chemical structure:
- The vast majority of GEP-NETs fall are further devided in two distinct categories:
- Despite great difference in hormone made and the action of these two , they are grouped together as GEP-NETs because of similarities in cell structure.[12]
- Pancreatic endocrine tumors (PETs) are also known as endocrine pancreatic tumors (EPTs) or islet cell tumors.
- further classification of gasterointestinal neuroendocrine tumors is based on:
- Carcinoid tumors (serotonin secreting) tend to grow much more slowly than PETs.
- In the context of GEP-NETs, the terms metastatic and malignant are often used interchangeably.
- GEP-NETs are often malignant, since the primary site often eludes detection for years, sometimes decades – during which time the tumor has the opportunity to metastasize.
- Researchers differ widely in their estimates of malignancy rates, especially at the level of the secretory subtypes (the various "-omas").
- The most common metastatic sites are the liver, the lymph nodes, and the bones.
- Liver metastases are so frequent and so well-fed that for many patients, they dominate the course of the cancer.
- For a patient with a nonsecretory PET, for example, the primary threat to life may be the sheer bulk of the tumor load in the liver.
Causes
- In the normal pancreas, islet cells produce hormones that regulate a variety of bodily functions, such as the control of blood sugar level and stomach acid production.
- Islet cell tumors include:
- The exact cause of neuroendocrine tumors is unknown.
- Different DNA mutations in neuroendocrine cells is supposed to be one of the causes of neuroendocrine tumors.
- Various hereditary syndromes associated with GIT and pancreatobiliary tract NETs are mentioned in detail in the table below:
| Name of the syndrome | Pattern of inheritance | Chromosomal Band Location | Gene/ Protein Involved | GIT and Pancreatobiliary Tract NETs | Other Tumors of GIT and Pancreatobiliary Tract | Clinical Presentation Outside GIT and Pancreatobiliary Tract |
|---|---|---|---|---|---|---|
| Multiple endocrine neoplasia 1 (MEN 1) |
|
|
Maybe associated with multiple: |
More commonly:
Less commonly:
| ||
| von Hippel-Lindau disease/syndrome (VHL) |
|
|
||||
| Neurofibromatosis 1 |
|
|||||
| Tuberous sclerosis |
|
| ||||
|
Other familial syndromes associated with neuroendocrine neoplasms are:
Epidemiology and Demographics
Lung NETs
- The portion of lung neuroendocrine tumors (NETs) is approximately 1 to 2% of lung malignancy in adults and 30% of neuroendocrine tumores.[13]
- Annual incidence of lung NETs was 1.49 per 100,000 population between 2000 and 2012 according to the United States Surveillance, Epidemiology, and End Results (SEER) database.
- Lung NETs are considered to be the most common primary lung neoplasm in children with typical presentation in late adolescence.[14][15][16][17]
- Average adult age at the time of diagnosis of a typical lung NET is 45 years, whereas patients with atypical tumors are approximately 10 years older.[18][19]
- Incidence of lung NETs differs from 0.2 to 2 per 100,000 population per year .
- There's a higher incidence of lung NETs in women as compared to men.
- Lung NET has annual incidence rates of 0.2 and 1.3 per 100,000 population among men and women respectively.
- White people have higher incidence of lung NETs as compared to black people.[20][21][13]
Digestive system NETs
- Annual incidence of digestive system NETs is 3.56 per 100,000 in United States approximately.
- Digestive system NENs of the tubular gastrointestinal tract and pancreas are relatively rare with an incidence of ≤1 case per 100,000 individuals per year..[13]
- Pancreatic NETs account for 1 to 2% (<3%) of all primary pancreatic neoplasms.[22][23][24][25]
- Following is a table that shows association of different diseases with the incidence of pancreatic NETs:
| Disease/Syndrome | % chance of developing Pancreatic NET |
|---|---|
| Multiple endocrine neoplasia 1 (MEN 1) | 80-100% |
| von Hippel-Lindau disease/syndrome (VHL) | 20% |
| Neurofibromatosis 1 | 10% |
| Tuberous sclerosis | 1% |
Risk factors
Few of the risk factors for neuroendocrine tumors include:
- Smoking is a risk factor for both lung and pancreatic NETs especially in case of atypical tumors.[20][26][27]
- Inherited predisposition is one of the risk factors especially in the case of lung NETs (not related to MEN syndrome).[28]
- Diabetes is associated with increased risk of pancreatic NETs.
- Chronic pancreatitis is associated with increased risk of pancreatic NETs.
- The inherited genetic syndromes associated with increased risk of causing neuroendocrine tumors include:
Natural History, Complications and Prognosis
- Neuroendocrine tumors are slow growing tumors but they can produce amino acids that can cause severe symptoms.
Complications
- Common complications of neuroendocrine tumors include the following:
- Diabetes
- Hormone crisis (if the tumor releases certain types of hormones)
- Severe low blood sugar (from insulinomas)
- Severe ulcers in the stomach and small intestine (from gastrinomas)
- Spread of the tumor to the liver
Prognosis
- Neuroendocrine tumors can be cured if they are removed surgically before their spread to the other organs.
- Chemotherapy can be used in case of cancerous tumors but it usually doesn't cure the patients.
- Life-threatening problems (such as a very low blood sugar level) can occur due to excess hormone production, or if cancer spreads throughout the body.
- Few of the prognostic facts about poorly differentiated pancreatic NEC are given below:[10]
- 88% of the patients have lymph node or distant metastatic disease at the time of tumor presentation.
- 7% of the patients subsequently develop metastases.
- The median survival is 11 months, ranging from 0 to 104 months.
- The two-year survival rate is 22.5%.
- The five-year survival rate is 16.1%.
History and Symptoms
- Initially, many of the neuroendocrine tumors mostly remain asymptomatic.
- When they cause symptoms, it is usually due to the following two reasons:
- Tumor location causing:
- Feeling of a growing lump under the skin
- Persistent pain occurring from increasing tumor size during its growth in specific area
- Unusually tired feeling (fatigue)
- Unintentional weight loss
- Loss of appetite
- Headache
- Jaundice
- Changes in bowel or bladder habits
- Unusual discharge or bleeding
- Persistent cough or hoarseness
- Persistent fever or night sweats
- Particular excessive hormone production by the tumor (functional tumor)
- Tumor location causing:
Carcinoid tumor
- Carcinoid tumors may produce serotonin (5-HT), a biogenic amine leading to the following specific set of symptoms which are collectively known as Carcinoid syndrome:
- Flushing of skin
- Diarrhea (or increase in the number of bowel movements)
- Weight loss
- Weight gain
- Palpitations
- Congestive heart failure (CHF)
- Asthma
- Acromegaly
- Cushing's syndrome
Pancreatic NETs
- According to the recent studies, 50 to 85% of the pancreatic NETs are nonfunctioning.
- Functioning pancreatic NETs include the following:
- Insulinomas with typical presentation of episodic hypoglycemia leading to:
- Confusion
- Amnesia (during hypoglycemic phase is common)
- Visual changes
- Palpitations
- Tremulousness
- Diaphoresis
- Unusual behavior
- Gastrinomas with typical presentation of:
- Peptic ulcer disease
- Diarrhea (prominent feature too sometimes)
- Glucagonoma causing classical clinical syndrome which includes:
- VIPoma syndrome presents with the following main clinical symptoms:
- Insulinomas with typical presentation of episodic hypoglycemia leading to:
Laboratory Findings
- Many neuroendocrine tumor cells especially possess strong receptors on their surfaces through which they receive hormonal messages such as:
- PETs often have strong receptors for somatostatin.
- Such tumor cells overexpress the somatostatin receptors (SSTRs) and are thus considered to be avid for this hormone, hence, their uptake of somatostatin is strong.
- This avidity for somatostatin is the key factor for the PETs' diagnosis and it also makes the tumors vulnerable to certain targeted therapies.
- Generally, the important common uses of tumor markers include:
CT Scan
- CT scan is one of the most common diagnostic tool used for diagnosing of Neuroendocrine tumors.
- CT scan with contrast medium can detect 95% of the tumors with size of >3 cm in size, and no tumors under 1 cm (University of Michigan Medical School n. d., [11]).
PET Scan
A gallium-68 receptor PET-CT, integrating a PET image with a CT image, is much more senstitive than an OctreoScan, and it generates objective (quantified) results in the form of a standardized uptake value (SUV).
Octreoscan
The diagnostic procedure that utilizes a somatostatin analog is the OctreoScan, also called somatostatin receptor scintigraphy (SRS or SSRS): a patient is injected with octreotide chemically bound to a radioactive substance, often indium-111; for those patients whose tumor cells are avid for octreotide, a radiation-sensitive scan can then indicate the locations of the larger lesions.
An OctreoScan is a relatively crude test that generates subjective results.
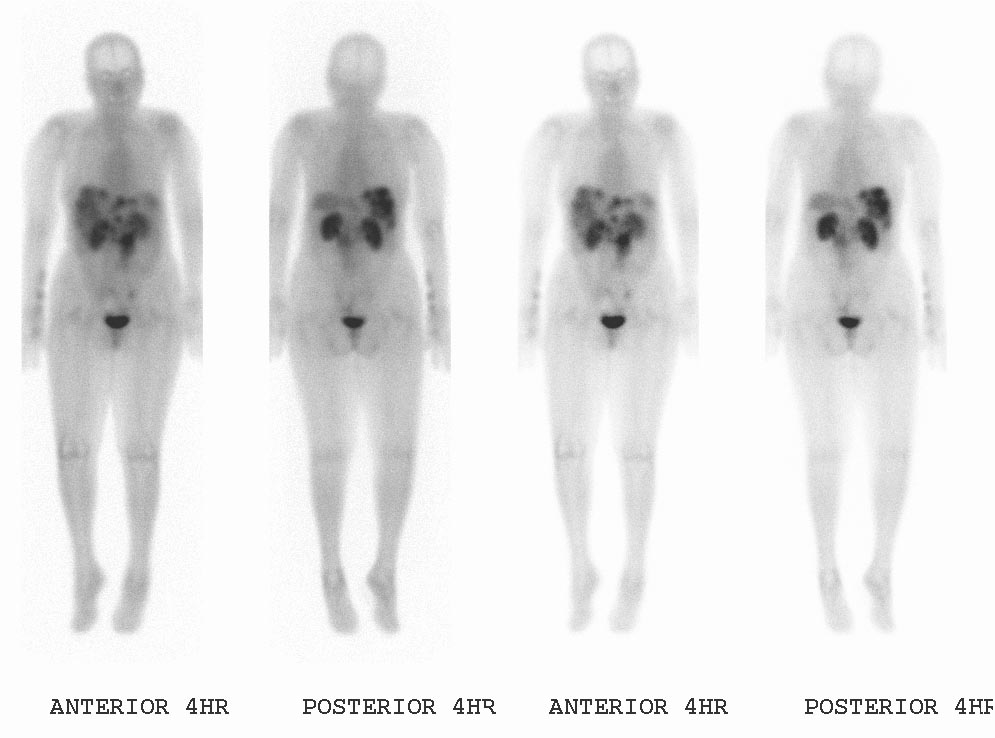
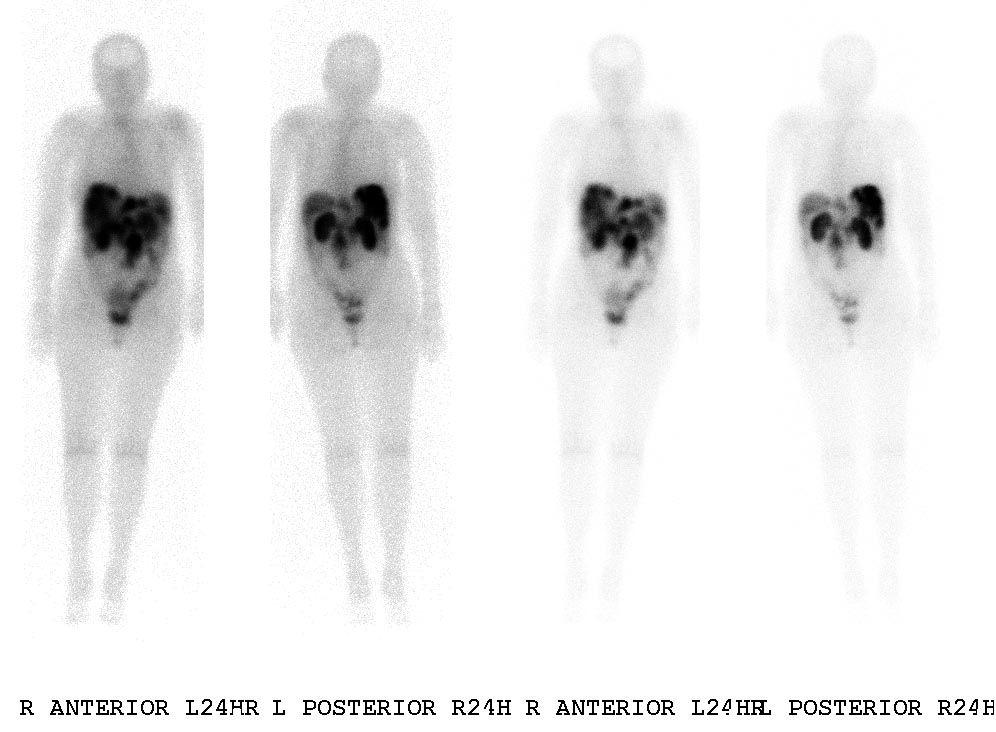
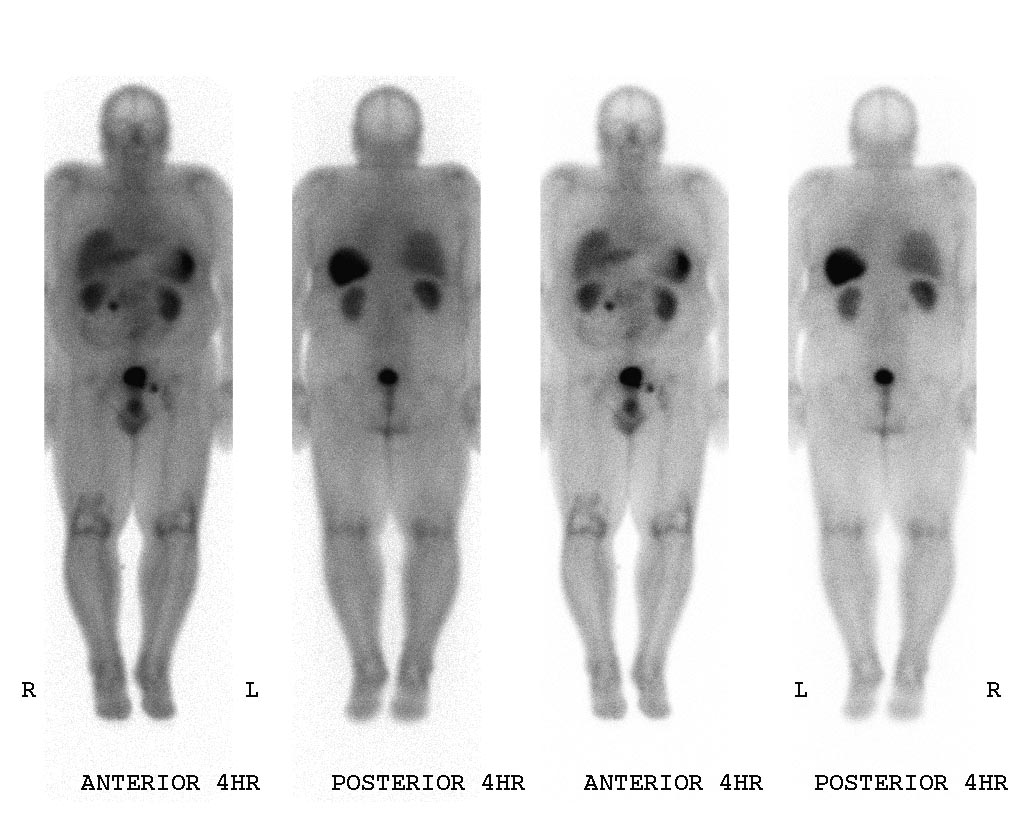

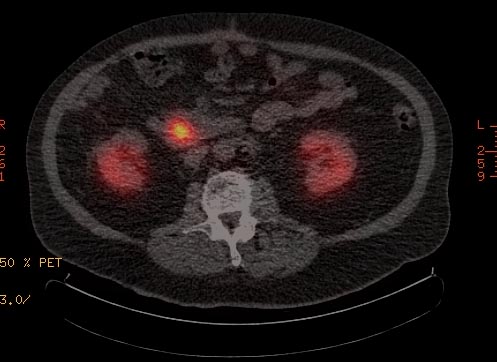
Medical Therapy
Approach
According to Warner, the best care, at least for noncarcinoid GEP-NETs, is provided by "an active [as opposed to wait-and-see] approach using sequential multimodality treatment" delivered by a "multidisciplinary team, which also may include a surgeon, endocrinologist, oncologist, interventional radiologist, and other specialists". This recommendation is based on his view that, except for most insulinomas, "almost all" PETs "have long-term malignant potential" – and in sixty percent of cases, that potential is already manifest. "Indeed, the most common cause of death from PETs is hepatic [that is, liver] failure" (Warner 2005, 4).
Two tricky issues in evaluating therapies are durability (is the therapy long-lasting?) and stasis (are the tumors neither growing nor shrinking?). For example, one therapy might give good initial results – but within months the benefit evaporates. And another therapy might be disparaged by some for causing very little tumor shrinkage, but be championed by others for causing significant tumoristasis.
The half-life of somatostatin in circulation is under three minutes, making it useless for diagnosis and targeted therapies. For this reason, The synthetic forms are typically called somatostatin analogs (somatostatin analogues), but according to the US Food and Drug Administration (FDA), the proper term is somatostatin congeners. (In this article we conform to the old terminology, as the medical community has been slow to adopt the term congener.) The analogs have a much longer half-life than somatostatin, and other properties that make them more suitable for diagnosis and therapy.
Chemotherapy
The most common nonsurgical therapy for all GEP-NETs is chemotherapy, although chemotherapy is reported to be largely ineffective for carcinoids, not particularly durable (long-lasting) for PETs, and inappropriate for PETs of nonpancreatic origin. [29]
When chemotherapy fails, the most common therapy, in the United States, is more chemotherapy, with a different set of agents. Some studies have shown that the benefit from one agent is not highly predictive of the benefit from another agent, except that the long-term benefit of any agent is likely to be low.
Strong uptake of somatostatin analogs is a negative indication for chemo.
Symptomatic relief
There are two major somatostatin-analog-based targeted therapies. The first of the two therapies provides symptomatic relief for patients with secretory tumors. In effect, somatostatin given subcutaneously or intramuscularly "clogs up" the receptors, blocking the secretion of hormones from the tumor cells. Thus a patient who might otherwise die from severe diarrhea caused by a secretory tumor can gain additional years of life.
Specific counter-hormones or other hormone-blocking medications are sometimes also used to provide symptomatic relief.
Hormone-delivered radiotherapy – PRRT
The second of the two major somatostatin-analog-based targeted therapies is called peptide receptor radionuclide therapy (PRRT), though we might simply call it hormone-delivered radiotherapy. In this form of radioisotope therapy (RIT), radioactive substances (called radionuclides or radioligands) are chemically conjugated with hormones (peptides or neuroamines); the combination is given intravenously to a patient who has good uptake of the chosen hormone. The radioactive labelled hormones enter the tumor cells, and the attached radiation damages the tumor- and nearby cells. Not all cells are immediately killed this way. The process of tumor cells dying as result of this therapy can go on for several months, even up to two years. In patients with strongly overexpressing tumor cells, nearly all the radiation either gets into the tumors or is excreted in urine. As Rufini et alia say, GEP-NETs "are characterized by the presence of neuroamine uptake mechanisms and/or peptide receptors at the cell membrane, and these features constitute the basis of the clinical use of specific radiolabeled ligands, both for imaging and therapy" (Rufini, Calcagni, and Baum 2006, [12]).
The use of PRRT for GEP-NETs is similar to the use of iodine-131 as a standard therapy (in use since 1943) for nonmedullary thyroid tumors (which are not GEP-NETs). Thyroid cells (whether normal or neoplastic) tend to be avid for iodine, and nearby cells are killed when iodine-131 is infused into the bloodstream and is soon attracted to thyroid cells. Similarly, overexpressing GEP-NET cells (neoplastic cells only) are avid for somatostatin analogs, and nearby cells are killed when radionuclides attached to somatostatin analogs are infused into the bloodstream and are soon attracted to the tumor cells. In both therapies, hormonal targeting delivers a much higher dose of radiation than external beam radiation could safely deliver.
As of 2006, PRRT is available in at least dozen medical centers in Europe. In the USA it is FDA-approved, and available at the MD Anderson Cancer Center, but using a radionuclide, indium-111, that is much weaker than the lutetium-177 and the even stronger yttrium-90 used on the European continent. In the UK, only the radionuclide metaiodobenzylguanidine (I-MIBG) is licensed (but GEP-NETs are rarely avid for MIBG). Most patients (from all over the world) are treated (with lutetium-177) in The Netherlands, at the Erasmus Medical Center. PRRT with lutetium or yttrium is nowhere an "approved" therapy, but the German health insurance system, for example, covers the cost for German citizens.
PRRT using yttrium or lutetium was first applied to humans about 1999. Practitioners continue to refine their choices of radionuclides to maximize damage to tumors, of somatostatin analogs to maximize delivery, of chelators to bind the radionuclides with the hormones (and chelators can also increase uptake), and of protective mechanisms to minimize damage to healthy tissues (especially the kidneys).
Hepatic artery-delivered therapies
- One therapy for liver metastases of GEP-NETs is hepatic artery embolization (HAE). Larry Kvols, of the Moffitt Cancer Center and Research Institute in Tampa, Florida, says that "hepatic artery embolization has been quite successful. During that procedure a catheter is placed in the groin and then threaded up to the hepatic artery that supplies the tumors in the liver. We inject a material called embospheres [tiny spheres of glass or resin, also called microspheres] into the artery and it occludes the blood flow to the tumors, and in more than 80% of patients the tumors will show significant tumor shrinkage" (Kvols 2002, [13]). HAE is based on the observation that tumor cells get nearly all their nutrients from the hepatic artery, while the normal cells of the liver get about 75 percent of their nutrients (and about half of their oxygen) from the portal vein, and thus can survive with the hepatic artery effectively blocked.
- Another therapy is hepatic artery chemoinfusion, the injection of chemotherapy agents into the hepatic artery. Compared with systemic chemotherapy, a higher proportion of the chemotherapy agents are (in theory) delivered to the lesions in the liver.
- Hepatic artery chemoembolization (HACE), sometimes called transarterial chemoembolization (TACE), combines hepatic artery embolization with hepatic artery chemoinfusion: embospheres bound with chemotherapy agents, injected into the hepatic artery, lodge in downstream capillaries. The spheres not only block blood flow to the lesions, but by halting the chemotherapy agents in the neighborhood of the lesions, they provide a much better targeting leverage than chemoinfusion provides.
- Radioactive microsphere therapy (RMT) combines hepatic artery embolization with radiation therapy – microspheres bound with radionuclides, injected into the hepatic artery, lodge (as with HAE and HACE) in downstream capillaries. This therapy is also called selective internal radiation therapy, or SIRT. In contrast with PRRT, the lesions need not overexpress peptide receptors. (But PRRT can attack all lesions in the body, not just liver metastases.) Due to the mechanical targeting, the yttrium-labeled microspheres "are selectively taken up by the tumors, thus preserving normal liver" (Salem et al. 2002, [14]).
Other Therapies
- Radiofrequency ablation (RFA) is used when a patient has relatively few metastases. In RFA, a needle is inserted into the center of the lesion and is vibrated at high frequency to generate heat; the tumor cells are killed by cooking.
- Cryoablation is similar to RFA; an endothermic substance is injected into the tumors to kill by freezing. Cryoablation has been considerably less successful for GEP-NETs than RFA.
- Interferon is sometimes used to treat GEP-NETs; its use was pioneered by Dr. Kjell Öberg at Uppsala. For GEP-NETs, Interferon is often used at low doses and in combination with other agents (especially somatostatin analogs such as octreotide). But some researchers claim that Interferon provides little value aside from symptom control.
- As described above, somatostatin analogs have been used for about two decades to alleviate symptoms by blocking the production of hormones from secretory tumors. They are also integral to PRRT. In addition, some doctors claim that, even without radiolabeling, even patients with nonsecretory tumors can benefit from somatostatin analogs, which purportedly can shrink or stabilize GEP-NETs. But some researchers claim that this "cold" octreotide provides little value aside from symptom control.
Surgery
Surgery is the only therapy that can cure GEP-NETs. However, the typical delay in diagnosis, giving the tumor the opportunity to metastasize, makes most GEP-NETs ineligible for surgery (non-resectable).
Case Studies
External links
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.
References
- ↑
"The APUD concept led to the belief that these cells arise from the embryologic neural crest. This hypothesis eventually was found to be incorrect" (Warner 2005, 2).
"The APUD-concept is currently abandoned" (Öberg 1998, 2, [1]).
- ↑ Tang LH, Basturk O, Sue JJ, Klimstra DS (2016). "A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas". Am J Surg Pathol. 40 (9): 1192–202. doi:10.1097/PAS.0000000000000662. PMC 4988129. PMID 27259015.
- ↑ Inzani F, Petrone G, Rindi G (2018). "The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia". Endocrinol Metab Clin North Am. 47 (3): 463–470. doi:10.1016/j.ecl.2018.04.008. PMID 30098710.
- ↑ 4.0 4.1 Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P; et al. (2013). "Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study". Ann Oncol. 24 (1): 152–60. doi:10.1093/annonc/mds276. PMID 22967994.
- ↑ Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G; et al. (2015). "Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms". Endocr Relat Cancer. 22 (4): 657–64. doi:10.1530/ERC-15-0119. PMID 26113608.
- ↑ Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM; et al. (2015). "The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms". Am J Surg Pathol. 39 (5): 683–90. doi:10.1097/PAS.0000000000000408. PMC 4398606. PMID 25723112.
- ↑ 7.0 7.1 Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L; et al. (2016). "Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas". Clin Cancer Res. 22 (4): 1011–7. doi:10.1158/1078-0432.CCR-15-0548. PMC 4988130. PMID 26482044.
- ↑ La Rosa S, Sessa F, Uccella S (2016). "Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms". Endocr Pathol. 27 (4): 284–311. doi:10.1007/s12022-016-9432-9. PMID 27169712.
- ↑ Shia J, Tang LH, Weiser MR, Brenner B, Adsay NV, Stelow EB; et al. (2008). "Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity?". Am J Surg Pathol. 32 (5): 719–31. doi:10.1097/PAS.0b013e318159371c. PMID 18360283.
- ↑ 10.0 10.1 Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM; et al. (2014). "Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases". Am J Surg Pathol. 38 (4): 437–47. doi:10.1097/PAS.0000000000000169. PMC 3977000. PMID 24503751.
- ↑ Oladejo AO (December 2009). "GASTROENTEROPANCREATIC NEUROENDOCRINE TUMORS (GEP-NETs) - APPROACH TO DIAGNOSIS AND MANAGEMENT". Ann Ib Postgrad Med. 7 (2): 29–33. PMC 4111010. PMID 25161467.
- ↑
"The main two groups of neuroendocrine GEP tumours are so-called carcinoid tumours and endocrine pancreatic tumours" (Öberg 2005a, 90, ).
"Less than 1% of carcinoids arise in the pancreas" (Warner 2005, 9).
Arnold et alia in effect define carcinoids as "extra-pancreatic endocrine gastronintestinal tumors" (Arnold et al. 2004, 196).
Some doctors believe that there is significant overlap between PETs and carcinoids. For example, endocrine surgeon Rodney Pommier says that "there are pancreatic carcinoids" (Pommier 2003, [2]). However, Pommier made his statement in a talk at a conference on carcinoids, not in a peer-reviewed journal; and in his talk he did not define the word carcinoid.
Another way to classify GEP-NETs is to separate those that begin in the glandular neuroendocrine system from those that begin in the diffuse neuroendocrine system. "Neuroendocrine tumors generally may be classified into two categories. The first category is an organ-specific group arising from neuroendocrine organs such as pituitary gland, thyroid, pancreas, and adrenal gland. The second group arises from the diffuse neuroendocrine cells/Kulchitsky cells that are widely distributed throughout the body and are highly concentrated in the pulmonary and gastrointestinal systems" (Liu et al. 2001, [3]).
- ↑ 13.0 13.1 13.2 Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y; et al. (2017). "Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States". JAMA Oncol. 3 (10): 1335–1342. doi:10.1001/jamaoncol.2017.0589. PMC 5824320. PMID 28448665.
- ↑ Quaedvlieg PF, Visser O, Lamers CB, Janssen-Heijen ML, Taal BG (2001). "Epidemiology and survival in patients with carcinoid disease in The Netherlands. An epidemiological study with 2391 patients". Ann Oncol. 12 (9): 1295–300. doi:10.1023/a:1012272314550. PMID 11697843.
- ↑ Modlin IM, Lye KD, Kidd M (2003). "A 5-decade analysis of 13,715 carcinoid tumors". Cancer. 97 (4): 934–59. doi:10.1002/cncr.11105. PMID 12569593.
- ↑ Hemminki K, Li X (2001). "Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden". Cancer. 92 (8): 2204–10. doi:10.1002/1097-0142(20011015)92:8<2204::aid-cncr1564>3.0.co;2-r. PMID 11596039.
- ↑ Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK; et al. (2008). "Neuroendocrine tumor epidemiology: contrasting Norway and North America". Cancer. 113 (10): 2655–64. doi:10.1002/cncr.23883. PMID 18853416.
- ↑ Skuladottir H, Hirsch FR, Hansen HH, Olsen JH (2002). "Pulmonary neuroendocrine tumors: incidence and prognosis of histological subtypes. A population-based study in Denmark". Lung Cancer. 37 (2): 127–35. PMID 12140134.
- ↑ Cao C, Yan TD, Kennedy C, Hendel N, Bannon PG, McCaughan BC (2011). "Bronchopulmonary carcinoid tumors: long-term outcomes after resection". Ann Thorac Surg. 91 (2): 339–43. doi:10.1016/j.athoracsur.2010.08.062. PMID 21256263.
- ↑ 20.0 20.1 Fink G, Krelbaum T, Yellin A, Bendayan D, Saute M, Glazer M; et al. (2001). "Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature". Chest. 119 (6): 1647–51. doi:10.1378/chest.119.6.1647. PMID 11399686.
- ↑ Gatta G, Ciccolallo L, Kunkler I, Capocaccia R, Berrino F, Coleman MP; et al. (2006). "Survival from rare cancer in adults: a population-based study". Lancet Oncol. 7 (2): 132–40. doi:10.1016/S1470-2045(05)70471-X. PMID 16455477.
- ↑ Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W; et al. (2010). "Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan". J Gastroenterol. 45 (2): 234–43. doi:10.1007/s00535-009-0194-8. PMID 20058030.
- ↑ Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K; et al. (2015). "Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis". J Gastroenterol. 50 (1): 58–64. doi:10.1007/s00535-014-0934-2. PMID 24499825.
- ↑ Ito T, Tanaka M, Imamura M, Neuroendocrine Tumor Workshop Japan (2008). "[Results of a nationwide survey of gastrointestinal tumors in Japan]". Nihon Geka Gakkai Zasshi. 109 (3): 128–32. PMID 18536315.
- ↑ Ito T, Tanaka M, Sasano H, Osamura YR, Sasaki I, Kimura W; et al. (2007). "Preliminary results of a Japanese nationwide survey of neuroendocrine gastrointestinal tumors". J Gastroenterol. 42 (6): 497–500. doi:10.1007/s00535-007-2056-6. PMID 17671766.
- ↑ Froudarakis M, Fournel P, Burgard G, Bouros D, Boucheron S, Siafakas NM; et al. (1996). "Bronchial carcinoids. A review of 22 cases". Oncology. 53 (2): 153–8. doi:10.1159/000227552. PMID 8604242.
- ↑ Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC (2008). "Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study". Int J Cancer. 123 (4): 867–73. doi:10.1002/ijc.23529. PMID 18491401.
- ↑ Oliveira AM, Tazelaar HD, Wentzlaff KA, Kosugi NS, Hai N, Benson A; et al. (2001). "Familial pulmonary carcinoid tumors". Cancer. 91 (11): 2104–9. doi:10.1002/1097-0142(20010601)91:11<2104::aid-cncr1238>3.0.co;2-i. PMID 11391591.
- ↑
Ramage et alia say that "response to chemotherapy in patients with strongly positive carcinoid tumours was of the order of only 10% whereas patients with SSRS negative tumours had a response rate in excess of 70%. The highest response rates with chemotherapy are seen in the poorly differentiated and anaplastic NETs: response rates of 70% or more have been seen with cisplatin and etoposide based combinations. These responses may be relatively short lasting in the order of only 8–10 months. Response rates for pancreatic islet cell tumours vary between 40% and 70% and usually involve combinations of streptozotocin (or lomustine), dacarbazine, 5-fluorouracil, and adriamycin. However, the best results have been seen from the Mayo clinic where up to 70% response rates with remissions lasting several years have been seen by combining chemoembolisation of the hepatic artery with chemotherapy. The use of chemotherapy for midgut carcinoids has a much lower response rate, with 15–30% of patients deriving benefit, which may only last 6–8 months (Ramage et al. 2005, [4]).
For 125 patients with histologically proven unresectable islet-cell carcinomas, "median duration of regression was 18 months for the doxorubicin combination and 14 months for the 5-FU combination" (Arnold et al. 2004, 230).
- ↑
"The liver gets about 80% of its blood and half the oxygen from the portal vein, and only 20% of the blood and the other 50% of the oxygen from the artery.... The liver gets 80% of its blood from the portal vein and 20% from that little hepatic artery. But tumors get 100% of their blood off the hepatic artery, and this has been shown by multiple lines of evidence (Pommier 2003, [5]).
"The normal liver gets its blood supply from two sources; the portal vein (about 70%) and the hepatic artery (30%)" (Fong and Schoenfield n. d., [6]).
- ↑ "The theoretical advantage is that higher concentrations of the agents can be delivered to the tumors without subjecting the patients to the systemic toxicity of the agents.... In reality, however, much of the chemotherapeutic agents does end up in the rest of the body" (Fong and Schoenfield, [7]).
- ↑ The "microspheres preferentially cluster around the periphery of tumor nodules with a high tumor:normal tissue ratio of up to 200:1". The SIRT-spheres therapy is not FDA-approved for GEP-NETs; "it is FDA approved for liver metastases secondary to colorectal carcinoma and is under investigation for treatment of other liver malignancies, such as hepatocellular carcinoma and neuroendocrine malignancies" (Welsh, Kennedy, and Thomadsen 2006, [8]).