Mitral stenosis
For patient information click here Template:DiseaseDisorder infobox
|
WikiDoc Resources for Mitral stenosis |
|
Articles |
|---|
|
Most recent articles on Mitral stenosis Most cited articles on Mitral stenosis |
|
Media |
|
Powerpoint slides on Mitral stenosis |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Mitral stenosis at Clinical Trials.gov Trial results on Mitral stenosis Clinical Trials on Mitral stenosis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Mitral stenosis NICE Guidance on Mitral stenosis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Mitral stenosis Discussion groups on Mitral stenosis Patient Handouts on Mitral stenosis Directions to Hospitals Treating Mitral stenosis Risk calculators and risk factors for Mitral stenosis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Mitral stenosis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
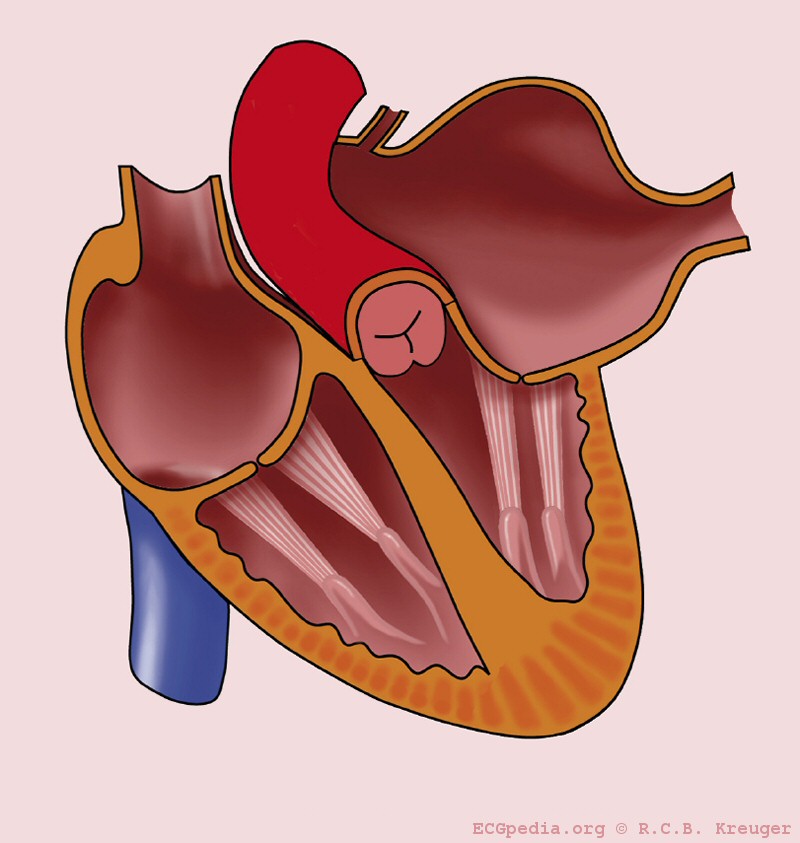
Mitral stenosis is a valvular heart disease characterized by the narrowing of the orifice of the mitral valve of the heart.
In normal cardiac physiology, the mitral valve opens during left ventricular diastole, to allow blood to flow from the left atrium to the left ventricle. Blood flows in the proper direction because during this phase of the cardiac cycle the pressure in the left ventricle is lower than the pressure in the left atrium, and the blood flows down the pressure gradient. In the case of mitral stenosis, the valve does not open completely, and to transport the same amount of blood the left atrium needs a higher pressure than normal to overcome the increased gradient.
Pathophysiology
Mitral stenosis (MS) is most commonly secondary to acute rheumatic fever. Generally, the initial valvulits is associated with valvular regurgitation but over a period of 2 or more years, the commissures fuse and the valves thicken and calcify. The chordal supporting structure also calcifies and retracts. The result is the typical “fish mouth deformity”. 70% of the time the mitral valve is involved in isolation, and 25% of the time the aortic valve is involved as well. The tricuspid and pulmonic valves are involved less commonly.
The normal mitral valve orifice area is 4-6 cm2. Mitral stenosis occurs when the orifice area is reduced to at least 2.2 cm2. This result in a gradient across the mitral valve which is the haemodynamic hallmark of the disease.
Differential Diagnosis of Causes of Mitral Stenosis
- Rheumatic Fever (RF)
- Systemic Lupus Erythematosus (SLE)
- Methysergide exposure
- Congenital “parachute” valve
- Obstruction to inflow by myxoma, cor triatriatum or a prosthetic valve can mimic the hemodynamics of mitral stenosis
- Amyloidosis
- Bacterial Endocarditis
- Congenital mitral stenosis
- Carcinoid Syndrome
- Heart tumor (myxoma)
- Rheumatoid Arthritis
Epidemiology and Demographics
There is a female predominance in the incidence of mitral stenosis as result of the underlying incidence among women rheumatic fever (RF).
Pathophysiology
The normal area of the mitral valve orifice is about 4 to 6 cm2. Under normal conditions, a normal mitral valve will not impede the flow of blood from the left atrium to the left ventricle during (ventricular) diastole, and the pressures in the left atrium and the left ventricle during diastole will be equal. The result is that the left ventricle gets filled with blood during early diastole, with only a small portion of extra blood contributed by contraction of the left atrium (the "atrial kick") during late ventricular diastole.
When the mitral valve area goes below 2 cm2, the valve causes an impediment to the flow of blood into the left ventricle, creating a pressure gradient across the mitral valve. This gradient may be increased by increases in the heart rate or cardiac output. As the gradient across the mitral valve increases, the amount of time necessary to fill the left ventricle with blood increases. Eventually, the left ventricle requires the atrial kick to fill with blood. As the heart rate increases, the amount of time that the ventricle is in diastole and can fill up with blood (called the diastolic filling period) decreases. When the heart rate goes above a certain point, the diastolic filling period is insufficient to fill the ventricle with blood and pressure builds up in the left atrium, leading to pulmonary congestion.
When the mitral valve area goes less than 1 cm2, there will be an increase in the left atrial pressures (required to push blood through the stenotic valve). Since the normal left ventricular diastolic pressures is about 5 mmHg, a pressure gradient across the mitral valve of 20 mmHg due to severe mitral stenosis will cause a left atrial pressure of about 25 mmHg. This left atrial pressure is transmitted to the pulmonary vasculature and causes pulmonary hypertension. Pulmonary capillary pressures in this level cause an imbalance between the hydrostatic pressure and the oncotic pressure, leading to extravasation of fluid from the vascular tree and pooling of fluid in the lungs (congestive heart failure causing pulmonary edema).
Increases in the heart rate will allow less time for the left ventricle to fill, also causing an increase in left atrial pressure and pulmonary congestion.
The constant pressure overload of the left atrium will cause the left atrium to increase in size. As the left atrium increases in size, it becomes more prone to develop atrial fibrillation. When atrial fibrillation develops, the atrial kick is lost (since it is due to the normal atrial contraction).
In individuals with severe mitral stenosis, the left ventricular filling is dependent on the atrial kick. The loss of the atrial kick due to atrial fibrillation can cause a precipitous decrease in cardiac output and sudden congestive heart failure.
Diagnosis
History and Symptoms
These reflect all of the anatomic, hemodynamic and compensatory mechanism at work. LA enlargement can be seen on chest x-ray (double-density of the left heart border, elevated left bronchus) and leads to dysphagia, hoarseness and the propensity for AF and thromboembolism. Impaired LV filling leads to fatigue. Increased LA pressure leads to pulmonary congestion and dyspnea, orthopnea and PND (paroxysmal nocturnal dyspnea ). Dilated bronchial veins can rupture leading to hemoptysis. PHTN (pulmonary hypertension) leads to RV (right ventricle) dysfunction, peripheral edema, pulsatile hepatomegaly etc.
Physical examination
The cardiac exam reflects the anatomy and hemodynamics. Initially, an OS is heard because there is an increased gradient between the LA and LV and S1 is loud. As the valve calcifies and LA pressure increases, S1 becomes softer and the OS moves closer to S2. When PHTN develops increased P2, pulmonary ejection sounds, murmurs of PI (Graham Steel), TR and right sided congestive heart failure (RVS3) can be heard. The diastolic murmur does not correlate with tee severity of MS but generally occurs throughout diastole in sever cases. Those in NSR (normal sinus rhythm) will have “presystolic accentuation” of the murmur due to atrial contraction.
Heart: The first heart sound is unusually loud and may be palpable due to the increased force of the closing of the mitral valve.
If pulmonary hypertension secondary to mitral stenosis is severe, the P2 (pulmonic) component of the second heart sound (S2) will become loud.
An opening snap which is a high pitched additional sound may be heard after the A2 (aortic) component of the second heart sound (S2), which correlates to the forceful opening of the mitral valve. The mitral valve opens when the pressure in the left atrium is greater than the pressure in the left ventricle. This happens in ventricular diastole (after closure of the aortic valve), when the pressure in the ventricle precipitously drops. In individuals with mitral stenosis, the pressure in the left atrium correlates with the severity of the mitral stenosis. As the severity of the mitral stenosis increases, the pressure in the left atrium increases, and the mitral valve opens earlier in ventricular diastole.
A mid-diastolic rumbling murmur will be heard after the opening snap. The murmur is best heard at the apical region and is not radiated. Since it is low-pitched it should be picked up by the bell of the stethoscope. Rolling the patient towards left, as well as isometric exercise will accentuate the murmur. A thrill might be present when palpating at the apical region of the praecordium.
Peripheral signs include:
- Malar flush - pulmonary hypertension is prominent in patients with mitral stenosis
- Ankle/sacral edema (oedema) when there is right heart failure
- Atrial fibrillation - irregular pulse and loss of 'a' wave in jugular venous pressure
- Left parasternal heave - presence of right ventricular hypertrophy due to pulmonary hypertension
- Tapping apex beat which is not displaced
Electrocardiographic Findings
-
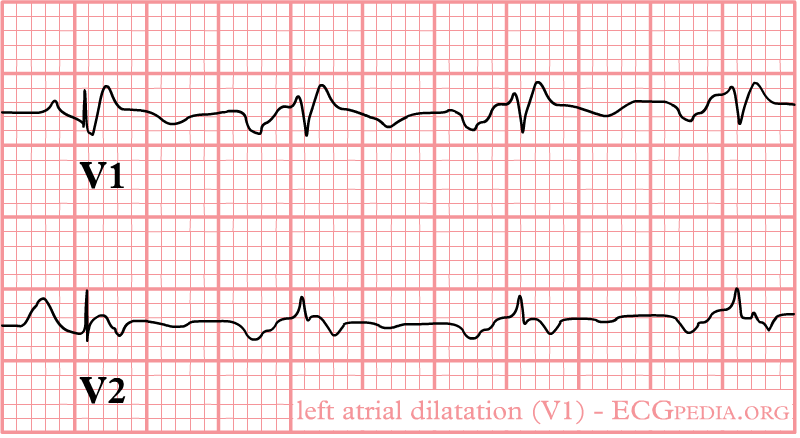
Left atrial enlargement
-
Left atrial enlargement as seen in lead V1.
-
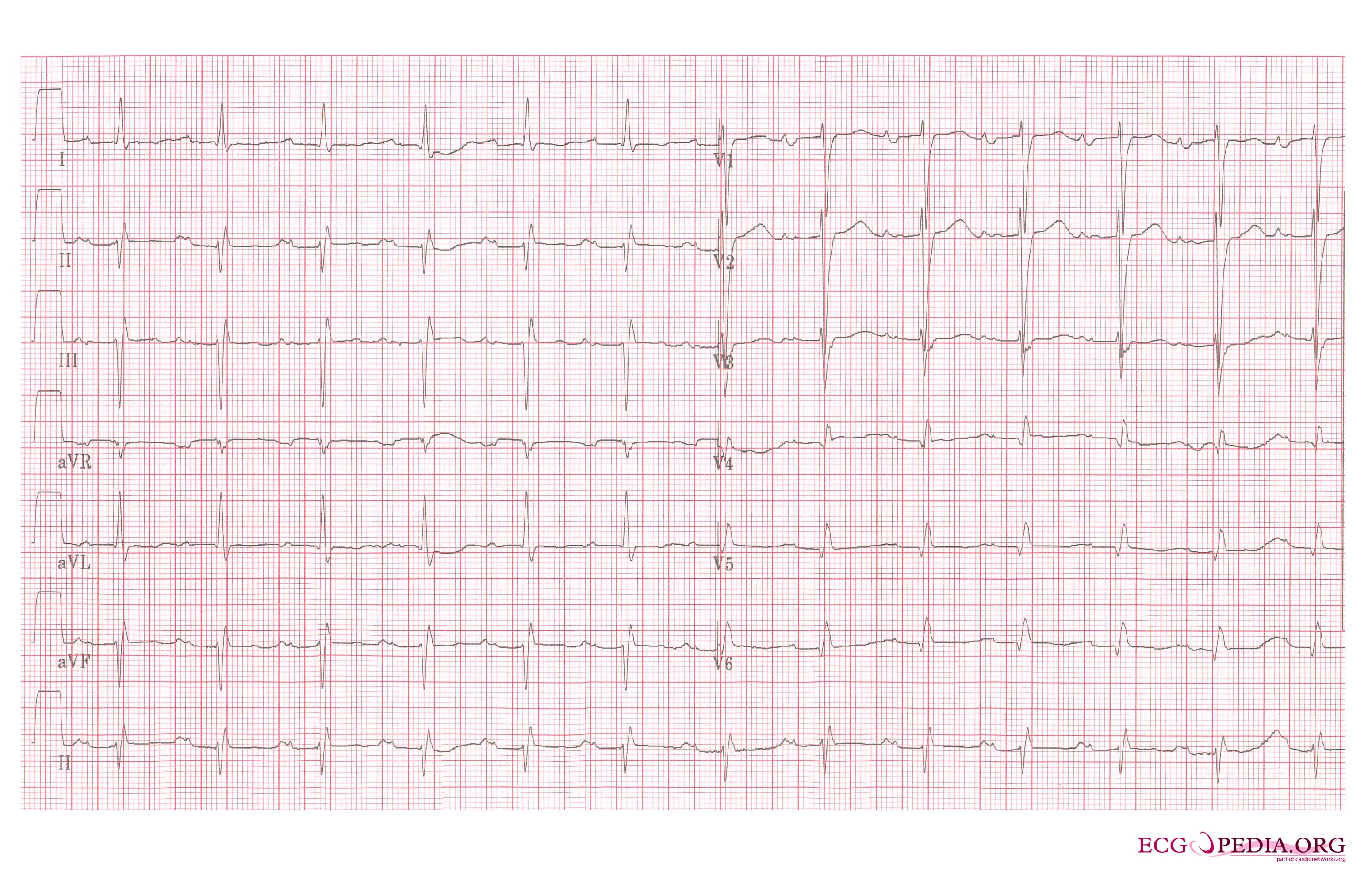
Left atrial enlargement, a 12 lead ECG
Imaging Studies
In most cases, the diagnosis of mitral stenosis is most easily made by echocardiography, which shows decreased opening of the mitral valve leaflets, and increased blood flow velocity during diastole. The trans-mitral gradient as measured by Doppler echocardiography is the gold standard in the evaluation of the severity of mitral stenosis.
| Degree of mitral stenosis | Mean gradient | Mitral valve area |
|---|---|---|
| Mild mitral stenosis | <5 | >1.5 cm2 |
| Moderate mitral stenosis | 5 - 10 | 1.0 - 1.5 cm2 |
| Severe mitral stenosis | > 10 | < 1.0 cm2 |
Chest X Ray
Left atrial enlargment are seen on chest x ray.
Echocardiographic Assessment
- ECHO is the standard and using various techniques the transmitral flows can be converted to valve areas.
- The MVA can be directly planimetered in some cases.
- ECHO findings also predict the success/failure of balloon valvuloplasty.
- Coexisting valve dysfunction can be identified as well as PHTN and RV dysfunction.
- Cardiac catheterization is a secondary modality used when surgical/percutaneous repair is contemplated or if symptoms are out of proportion to noninvasive testing results.
Mitral valve assessment with echocardiography should include:
- Diagnosis from the pattern of valve involvement and calcification.
- severity of mitral stenosis
- Associated mitral regurgitation
- Other co-existent valve lesions
- Chamber dilatation and function
M-mode echocardiography
M-mode echocardiographic assessment of the valve reveals slow early diastolic closure of the mitral valve. The mid-diastolic closure velocity or E-F slope is remarkably reduced. This can be used to assess the severity of the mitral stenosis and to determine re-stenosis from serial measurements after surgical or percutaneous treatment. E-F slope can also be flat in subjects with normal mitral valve if the left ventricular compliance is reduced.
Another M-mode feature of mitral stenosis is the anterior movement of posterior mitral valve leaflet in early diastole.
2D-Echocardiography
As with any stenotic valve, the main diagnostic feature in the parasternal long axis view is the doming of the anterior mitral valve leaflet in diastole. This is due to the reduced mobility of the valve tips compared to the base of the leaflets.
Thickening of the valve leaflets with or without calcification can be visualized with echocardiography. This can also involve the annulus and the chordae which can be shortened.
Other associated features may include markedly enlarged left atrium, pulmonary hypertension, right heart enlargement and tricuspid regurgitation. There may be involvement of other valves as well.
Orifice area by planimetry
- A well validated technique for assessing severity
- In parasternal short axis view
- The mitral valve is funnel shaped, so the area needs to be measured at the tip of the valves (the narrowest portion).
- Be sure to turn the gain down to have low overall 2D gain.
- Trace the inner edge of the valve orifice during the maximum opening in diastole.
- Not useful if heavily calcified valves or after valvotomy
- Sometimes chordae can mimic the valve orifice.
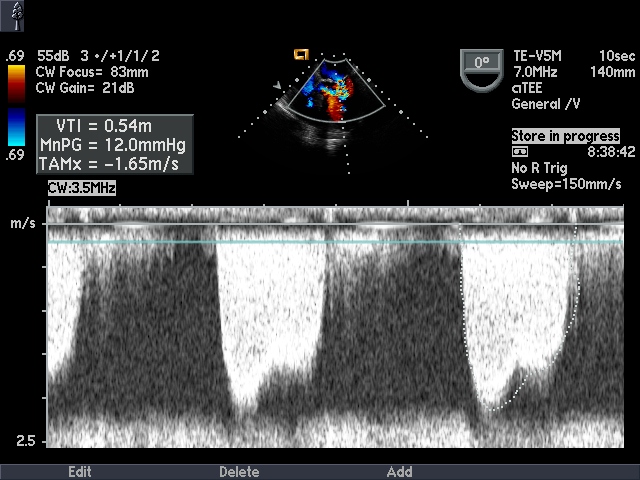
Doppler echocardiography
Mean transmitral valve gradient
Can be measured by tracing the outline of mitral diastolic inflow and the mean pressure gradient is automatically calculated. The severity can be assessed as mild (<5), moderate (5-10) and severe (>10).
Pressure half time
The rate of pressure decline across the stenotic orifice is determined by the cross sectional area of the orifice. Smaller the orifice leads to slower rate of pressure decline.
Pressure half time is defined as the time interval between maximum early diastolic pressure gradient and the point at which the pressure gradient is half the maximum value.
By echocardiography, this is measured in apical-4-chamber view with continuous wave Doppler aligned with the inflow jet of mitral valve. The spectral trace is recorded and the slope of the flow is measured from the beginning of E-wave ignoring the A-wave.
Based on the Bernoulli equation, the velocity of pressure half time can be derived as V1/2 = 0.7 Vmax
The mitral valve area can be calculated as MV area (cm2) = 220/Pressure half time (msec)
Limitations of pressure half time
- Assumes normal left atrial and left ventricular compliance. This is not true immediately post valvuloplasty (72 hrs) and in left ventricular hypertrophy
- Aortic regurgitation leads to increased left ventricular diastolic pressure and thus shorter pressure half time. Hence Mitral valve area is over estimated.
- Angle needs constant intercept angle parallel to the flow
- Atrial fibrillation - several beats need to be averaged.
- Atrial septal defect - left to right shunt, shortened pressure half time and hence mitral valve area over estimated.
- Mitral regurgitation does not affect pressure half time.
Continuity equation Mitral valve area
Mitral valve area = transmitral stroke volume / velocity time integrale of the MS jet.
- M-Mode Echo
 |
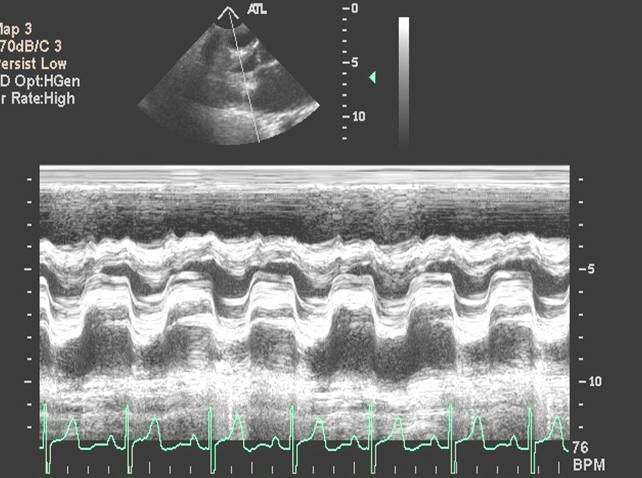 |
- Continuous Wave Doppler Echo
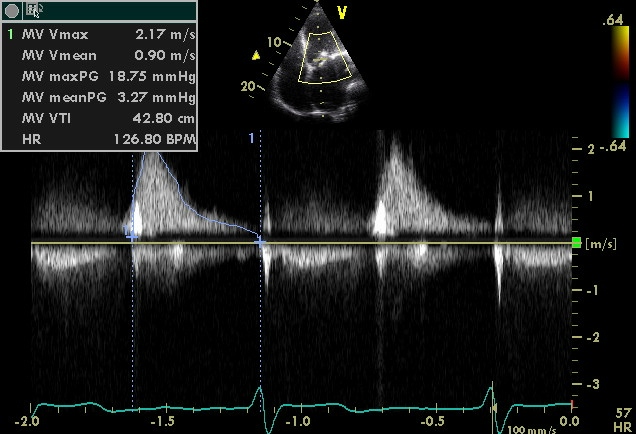
- 3-D Echo of Rheumatic Mitral Stenosis 1
<googlevideo>-3098493179966000395&hl=en</googlevideo>
- 3-D Echo of Rheumatic Mitral Stenosis 2
<googlevideo>-1233084670908574806&hl=en</googlevideo>
- 3-D Echo of Rheumatic Mitral Stenosis 3
<googlevideo>4388526645575160977&hl=en</googlevideo>
- Calcific Mitral Stenosis Continuous Wave Doppler
- Calcific Mitral Stenosis Severe 1
<googlevideo>-2694339846253882313&hl=en</googlevideo>
- Calcific Mitral Stenosis Severe 2
<googlevideo>-4939554452635824325&hl=en</googlevideo>
- Calcific Mitral Stenosis Severe 3
<googlevideo>-4939554452635824325&hl=en</googlevideo>
- Calcific Mitral Stenosis Severe 4
<googlevideo>9174885547972304289&hl=en</googlevideo>
- Calcific Mitral Stenosis Severe 5
<googlevideo>451524906007806928&hl=en</googlevideo>
Assessment of severity
| Severity | mild | moderate | severe |
|---|---|---|---|
| Mitral valve area | 2.2 - 1.5 | 1- 1.15 | <1 |
| Pressure Half time (msec) | 100 - 150 | 150 - 220 | >220 |
| Mean Pressure Gradient | <5 | 5-10 | >10 |
| TR velocity | <2.7 | >3 | |
| Pulmonary artery pressure | <30 | >50 |
Factors favouring successful percutaneous mitral valvuloplasty
Mitral stenosis is amenable to percutaneous mitral valvuloplasty if the echocardiography demonstrates :
- Thickening confined to valve tips
- Good mobility of Anterior mitral valve leaflet
- Little chordal involvement
- not more than trivial mitral regurgitation
- no left atrial thrombus
- no commissural calcification.
Wilkins score
A scoring system exists to grade the morphological changes in the mitral valve during assessment with echocardiography. This takes into account 4 characteristics: leaflet mobility, leaflet thickening, valve calcification and involvement of the subvalvular apparatus. The involvement is graded from 0-4. A total score of more than 8 is predictive of a low success post percutaneous mitral valvuloplasty.[1]
Cardiac Catheterization
A definitive method of assessing the severity of mitral stenosis is the simultaneous left heart catheterization and right heart catheterization. The right heart catheterization gives the physician the mean pulmonary capillary wedge pressure, which is a reflection of the left atrial pressure. The left heart catheterization, on the other hand, gives the pressure in the left ventricle. By simultaneously taking these pressures, it is possible to determine the gradient between the left atrium and right atrium during ventricular diastole, which is a marker for the severity of mitral stenosis. This method of evaluating mitral stenosis tend to over-estimate the degree of mitral stenosis, however, because of the time lag in the pressure tracings seen on the right heart catheterization and the slow Y descent seen on the wedge tracings. If a trans-septal puncture is made during right heart catheterization, however, the pressure gradient can accurately quantify the severity of mitral stenosis.
Natural history
The natural history of mitral stenosis secondary to rheumatic fever (the most common cause) is an asymptomatic latent phase following the initial episode of rheumatic fever. This latent period lasts an average of 16.3 ± 5.2 years. Once symptoms of mitral stenosis begin to develop, progression to severe disability takes 9.2 ± 4.3 years.
In individuals who were offered mitral valve surgery but refused, survival with medical therapy alone was 44 ± 6% at 5 years, and 32 ± 8% at 10 years after they were offered correction.
Treatment
The treatment options for mitral stenosis include medical management, surgical replacement of the valve, and percutaneous balloon valvuloplasty.
Mitral stenosis typically progresses slowly (over decades) from the initial signs of mitral stenosis to NYHA functional class II symptoms to the development of atrial fibrillation to the development of NYHA functional class III or IV symptoms. Once an individual develops NYHA class III or IV symptoms, the progression of the disease accelerates and the patient's condition deteriorates.
Pharmacotherapy
- In asymptomatic patients, use endocarditis prophylaxis and chronic anticoagulation for intermittent or chronic atrial fibrillation, systemic embolism and marked LA enlargement (>55mm).
- In symptomatic patients, control heartrate and Maintain NSR (normal sinus rhythm) (digoxin, antiarrhythmic agents) and B-blockers. Use diuretics for control of pulmonary edema. The decision of whether to proceed with vavluloplasty or surgical commissurotomy depends on the severity of symptoms and/or severe (>50mm Hg) PHTN. Relative indications would be for Class II, III symptoms, episodic pulmonary edema, prevention of thromboembolism, or moderate (45-50mm Hg) PHTN. The decision of whether valvuloplasty is superior to surgery depends on age (<60 favors valvuloplasty), and Cath/ECHO findings (e.g. LVEDP, degree of mobility, thickening and calcification). The average end result with both strategies is about 2 cm2. Moderate or greater MR (mitral regurgitation) and LA thrombus are contraindications to valvuloplasty.
Surgery and Device Based Therapy
Indications for Mitral Valvuloplasty
Associate Editor-In-Chief: Joanna J. Wykrzykowska, MD Contact at [4]; Phone: 617-767-5343 and Roger J. Laham, MD Contact at [5]
Patient selection
- Mitral stenosis due to rheumatic disease is becoming less common in the US but is very prevalent worldwide
- Symptoms of shortness of breath and valve area or less than 1.5 cm2 are indications for commissurotomy
- Unlike with the surgical approach, elevated pulmonary pressures or depressued LV function are not contraindications
- Wilkins score that describes valve anatomy is the best predictor of procedural success: it assigns points for leaflet mobility, valvular and subvulvular thickening and calcification degree (score of < 8 makes the patient a favorable candidate); Thus good quality echocardiogram is essential before qualifying the patient for the procedure
- Contraindications include presence of left atrial appendage clot, moderate to severe mitral regurgitation or other indications for open heart surgery
Technique
- Transvenous transeptal technique is most commonly used with the Inoue balloon system
- Fossa ovalis lies usually at 1-7 o’clock but this orientation can be distorted in the presence of mitral stenosis where the interatrial septum becomes more flat, horizontal and lower
- For the femoral vein approach a 70 cm Brockenbrough needle should be used or an 8 Fr Mullins sheath and advanced under fluoroscopic guidance with pressure monitoring
- The latter is necessary to monitor for puncture into adjacent structures such as aorta
- Further catheter manipulation may be necessary to direct the catheter into the left ventricle through the mitral valve rather than towards one of the pulmonary veins
- The Mullins sheath is exchanged for a solid-core coiled 0.025 inch guidewire over which a 14 Fr dilator is placed
- This is exchanged for the Inoue balloon (24-30 mm) which inflates in three stages allowing for balloon self-positioning with the last inflation resulting in commissural splitting
Hemodynamic and Clinical Outcomes
- Results of the commissurotomy should be assessed with hemodynamics and echocardiography
- If second inflation is needed mitral regurgitation should be assessed
- In general increasing valve area to greater than 1 cm2/m2 is an acceptable result
- Usually the valve area doubles and the pulmonary pressures degrease immediately
- 5 year survival is in the 90% range
Complications:
- Usually less than 5% with low mortality
- Failure to puncture the interatrial septum is the most common reason for aborted procedure
- Most common complication is development of severe mitral regurgitation The indication for invasive treatment with either a mitral valve replacement or valvuloplasty is NYHA functional class III or IV symptoms.
To determine which patients would benefit from percutaneous balloon mitral valvuloplasty, a scoring system has been developed.2 Scoring is based on 4 echocardiographic criteria: leaflet mobility, leaflet thickening, subvalvar thickening, and calcification. Individuals with a score of ≥ 8 tended to have suboptimal results.3 Superb results with valvotomy are seen in individuals with a crisp opening snap, score < 8, and no calcium in the commissures.
See also
External links
- Echocardiography in Mitral stenosis at Wikiecho
- Echocardiographic features of Mitral Stenosis
- Mitral Valve Repair at The Mount Sinai Hospital
References
- Bonow RO, Carabello B, de Leon AC; et al. (1998). "ACC/AHA guidelines for the management of patients with valvular heart disease". J. Am. Coll. Cardiol. 32 (5): 1486–588. PMID 9809971.
- Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF (1988). "Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation". British heart journal. 60 (4): 299–308. PMID 3190958.
- Abascal VM, Wilkins GT, O'Shea JP; et al. (1990). "Prediction of successful outcome in 130 patients undergoing percutaneous balloon mitral valvotomy". Circulation. 82 (2): 448–56. PMID 2372892.
de:Mitralstenose it:Stenosi mitralica nn:Mitralstenose sv:Mitralisstenos
- ↑ Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308. doi: 10.1136/hrt.60.4.299