Migalastat
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Migalastat is an alpha-galactosidase A (alpha-Gal A) pharmacological chaperone that is FDA approved for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant based on in vitro assay data. Common adverse reactions include headache, nasopharyngitis, urinary tract infection, nausea, and pyrexia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Migalastat is indicated for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant based on in vitro assay data.
- This indication is approved under accelerated approval based on reduction in kidney interstitial capillary cell globotriaosylceramide (KIC GL-3) substrate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Dosage
- The recommended dosage regimen of migalastat is 123 mg orally once every other day at the same time of day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding migalastat Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding migalastat Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and effectiveness of migalastat have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding migalastat Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding migalastat Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
There is limited information regarding Migalastat Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In clinical trials, 139 patients with Fabry disease (79 females, 60 males, 92% Caucasian, ages 16 to 72 years), who were naïve to migalastat or previously treated with enzyme replacement therapy, were exposed to at least one dose of migalastat. Of the 139 patients, 127 patients were exposed to migalastat 123 mg every other day for 6 months and 123 patients were exposed for greater than one year. The clinical trials included one randomized, double-blind, placebo-controlled clinical trial of 6 months duration followed by a 6-month open-label treatment phase (Study 1). A second trial was a randomized, open-label, active-controlled clinical trial of 18 months duration in patients with Fabry disease receiving enzyme replacement therapy who were randomized to either switch to migalastat or continue enzyme replacement therapy (Study 2; NCT01218659). In addition, there were two open-label, long-term extension trials.
- The most common adverse reactions reported with migalastat (≥ 10%) during the 6-month placebo-controlled, double-blind phase of Study 1 were headache, nasopharyngitis, urinary tract infection, nausea, and pyrexia.
- TABLE 1 shows adverse reactions reported in at least 5% of patients treated with migalastat (and at a higher rate than placebo) during the 6-month placebo-controlled, double-blind phase of Study 1.
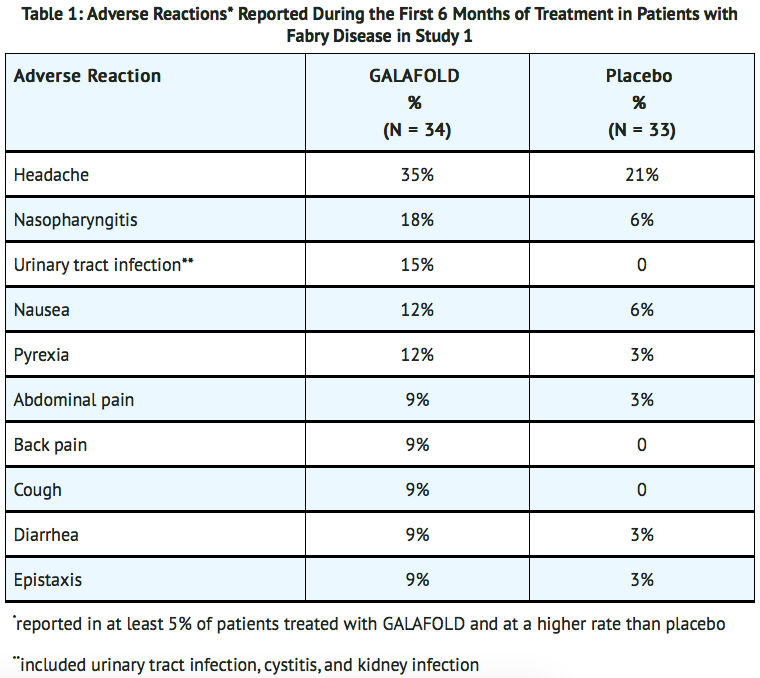
- Adverse reactions reported in > 5% of patients who received migalastat in the 6-month open-label treatment phase of Study 1, in Study 2, and in the long-term extension trials (N = 115, mean duration of treatment 2.7 years) included those reported in TABLE 1 with the addition of vomiting.
Postmarketing Experience
There is limited information regarding Migalastat Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Migalastat Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There were three pregnant women with Fabry disease exposed to migalastat in clinical trials. As such, the available data are not sufficient to assess drug associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, no adverse developmental effects were observed.
- The estimated background risk for major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
- No adverse developmental effects were observed with oral administration of migalastat to pregnant rats and rabbits during organogenesis at doses up to 26 and 54 times, respectively, the recommended dose based on AUC. No effects on post-natal development were observed following oral administration of up to 500 mg/kg migalastat twice daily to pregnant rats (16 times the recommended dose based on AUC) during organogenesis and through lactation.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Migalastat in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Migalastat during labor and delivery.
Nursing Mothers
Risk Summary
- There are no human data available on the presence of migalastat in human milk, the effects on the breastfed infant, or the effects on milk production. Migalastat is present in the milk of lactating rats (see DATA). When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for migalastat and any potential adverse effects on the breastfed child from migalastat or from the underlying maternal condition.
Animal Data
- Migalastat concentrations in milk from rats following oral administration of up to 500 mg/kg twice daily (approximately 16 times the recommended human dose based on AUC) was approximately 2.5 times higher than levels in the rat maternal plasma at 4 hours post-dose. The concentration of migalastat in plasma from pups was approximately 11 times lower than the maternal plasma concentrations at 1 hour post-dose.
Pediatric Use
- The safety and effectiveness of migalastat have not been established in pediatric patients.
Geriatic Use
- Clinical trials of migalastat did not include a sufficient number of patients 65 years and older to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Migalastat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Migalastat with respect to specific racial populations.
Renal Impairment
- Migalastat is substantially excreted by the kidneys. Systemic exposure was significantly increased in subjects with severe renal impairment (eGFR less than 30 mL/min/1.73 m2). Migalastat has not been studied in patients with Fabry disease who have an eGFR less than 30 mL/min/1.73 m2. Migalastat is not recommended for use in patients with severe renal impairment or end-stage renal disease requiring dialysis. No dosage adjustment is required in patients with mild to moderate renal impairment (eGFR at least 30 mL/min/1.73 m2 and above).
Hepatic Impairment
There is no FDA guidance on the use of Migalastat in patients with hepatic impairment.
Females of Reproductive Potential and Males
Infertility
- The effects of migalastat on fertility in humans have not been studied. Transient and fully reversible infertility in male rats was associated with migalastat treatment at a systemic exposure (AUC) equivalent to the human exposure at the recommended dose. Complete reversibility was seen at 4 weeks after the termination of treatment. Migalastat did not affect fertility in female rats.
Immunocompromised Patients
There is no FDA guidance one the use of Migalastat in patients who are immunocompromised.
Administration and Monitoring
Administration
- Select adults with confirmed Fabry disease who have an amenable GLA variant for treatment with migalastat.
- Treatment is indicated for patients with an amenable GLA variant that is interpreted by a clinical genetics professional as causing Fabry disease (pathogenic, likely pathogenic) in the clinical context of the patient. Consultation with a clinical genetics professional is strongly recommended in cases where the amenable GLA variant is of uncertain clinical significance (VUS, variant of uncertain significance) or may be benign (not causing Fabry disease).
- The recommended dosage regimen of migalastat is 123 mg orally once every other day at the same time of day.
- Take migalastat on an empty stomach. Do not consume food at least 2 hours before and 2 hours after taking migalastat to give a minimum 4 hours fast. Clear liquids can be consumed during this 4-hour period.
- Do not take migalastat on 2 consecutive days.
- If a dose is missed entirely for the day, take the missed dose of migalastat only if it is within 12 hours of the normal time that the dose should have been taken. If more than 12 hours have passed, resume taking migalastat at the next planned dosing day and time, according to the every-other-day dosing schedule.
- Swallow capsules whole. Do not cut, crush, or chew.
Monitoring
There is limited information regarding Migalastat Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Migalastat and IV administrations.
Overdosage
There is limited information regarding Migalastat overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Migalastat
| |
| Systematic (IUPAC) name | |
| (2R,3S,4R,5S)-2-(Hydroxymethyl)-3,4,5-piperidinetriol | |
| Identifiers | |
| CAS number | 75172-81-5 (HCl) |
| ATC code | A16 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| SMILES | & |
| Synonyms | DDIG, AT1001 |
| Pharmacokinetic data | |
| Bioavailability | 75% |
| Protein binding | None |
| Metabolism | ? |
| Half life | 3–5 hours (single dose) |
| Excretion | Urine (77%), feces (20%) |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
Insufficient data |
| Legal status |
Template:Unicode Prescription only |
| Routes | By mouth (capsules) |
Mechanism of Action
- Migalastat is a pharmacological chaperone that reversibly binds to the active site of the alpha-galactosidase A (alpha-Gal A) protein (encoded by the galactosidase alpha gene, GLA), which is deficient in Fabry disease. This binding stabilizes alpha-Gal A allowing its trafficking from the endoplasmic reticulum into the lysosome where it exerts its action. In the lysosome, at a lower pH and at a higher concentration of relevant substrates, migalastat dissociates from alpha-Gal A allowing it to break down the glycosphingolipids globotriaosylceramide (GL-3) and globotriaosylsphingosine (lyso-Gb3). Certain GLA variants (mutations) causing Fabry disease result in the production of abnormally folded and less stable forms of the alpha-Gal A protein which, however, retain enzymatic activity. Those GLA variants, referred to as amenable variants, produce alpha-Gal A proteins that may be stabilized by migalastat thereby restoring their trafficking to lysosomes and their intralysosomal activity.
In Vitro Amenability Assay
- In an in vitro assay (HEK-293 assay), Human Embryonic Kidney (HEK-293) cell lines were transfected with specific GLA variants (mutations) which produced mutant alpha-Gal A proteins. In the transfected cells, amenability of the GLA variants was assessed after a 5-day incubation with 10 micromol/L migalastat. A GLA variant was categorized as amenable if the resultant mutant alpha-Gal A activity (measured in the cell lysates) met two criteria: 1) it showed a relative increase of at least 20% compared to the pre-treatment alpha-Gal A activity, and 2) it showed an absolute increase of at least 3% of the wild-type (normal) alpha-Gal A activity.
- The in vitro assay did not evaluate trafficking of the mutant alpha-Gal A proteins into the lysosome or the dissociation of migalastat from the mutant alpha-Gal A proteins within the lysosome. Also, the in vitro assay did not test whether a GLA variant causes Fabry disease or not.
- The GLA variants which are amenable to treatment with migalastat, based on the in vitro assay data, are shown in TABLE 2. Inclusion of GLA variants in this table does not reflect interpretation of their clinical significance in Fabry disease. Whether a certain amenable GLA variant in a patient with Fabry disease is disease-causing or not should be determined by the prescribing physician (in consultation with a clinical genetics professional, if needed) prior to treatment initiation. Consultation with a clinical genetics professional is strongly recommended in cases where the amenable GLA variant is of uncertain clinical significance (VUS, variant of uncertain significance) or may be benign (not causing Fabry disease).

- If a GLA variant does not appear in TABLE 2, it is either non-amenable (if tested) or has not been tested for in vitro amenability. For further information, please contact Amicus Medical Information at 1-877-4AMICUS or MEDINFOUSA@AMICUSRX.COM.
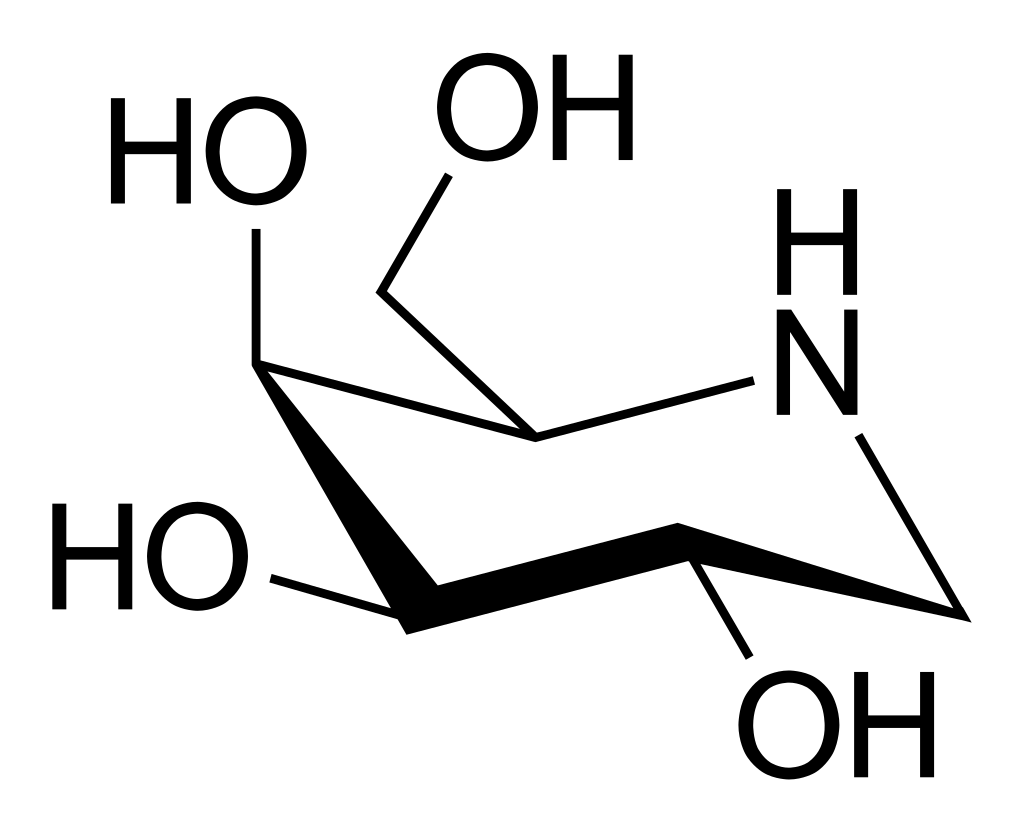
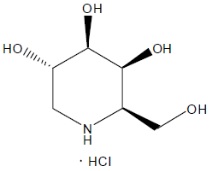
Structure
- Its molecular formula is C6H13NO4•HCl, molecular mass is 199.63 g/mol, and its chemical structure is depicted below.
Pharmacodynamics
- In Study 1, 31 of 50 patients with amenable GLA variants (18 on migalastat, 13 on placebo) had lyso-Gb3 assessments available after 6 months of treatment. The median change from baseline to month 6 in plasma lyso-Gb3 (nmol/L) was -2.37 (range -69.7, 1.8) in patients on migalastat and 0.53 (range -21.5, 16.3) in patients on placebo. In the open-label treatment phase of Study 1, the 13 patients who were initially on placebo for 6 months and who switched to migalastat for another 6 months had a median change in lyso-Gb3 (nmol/L) of -2.72 (range -61.1, -0.3) . The 18 patients who were treated with migalastat for 6 months and then continued migalastat in the open-label treatment phase of Study 1 for an additional 6 months had no further changes in plasma lyso-Gb3.
- In Study 2, 46 of 56 patients with amenable GLA variants (31 on migalastat, 15 on enzyme replacement therapy (ERT)) had lyso-Gb3 assessments available after 18 months of treatment. The median change from baseline to month 18 in plasma lyso-Gb3 (nmol/L) was 0.53 (range -2.27, 28.3) in patients on migalastat and -0.03 (range -11.9, 2.57) in patients on ERT.
Cardiac Electrophysiology
- At a dose approximately 8 times the recommended dose, migalastat did not prolong the QT interval to any clinically relevant extent.
Pharmacokinetics
Absorption
- Following a single migalastat oral dose of 123 mg, the absolute bioavailability (AUC) of migalastat was approximately 75% and the time to peak plasma concentration was approximately 3 hours. Plasma migalastat exposure (AUC0-∞ and Cmax) demonstrated dose-proportional increases at oral doses from 75 mg to 1250 mg (doses from 0.5 to 8.3-fold of the approved recommended dosage). Migalastat does not accumulate following administration of 123 mg migalastat every other day.
Effect of Food
- Administration of migalastat one hour before a high-fat (850 calories; 56% from fat) or light meal (507 calories; 30% from fat), or one hour after a light meal, reduced the mean migalastat AUC0-∞ by 37% to 42% and Cmax by 15% to 39% compared to the fasting state.
Distribution
- The apparent volume of distribution (Vz/F) of migalastat in Fabry patients was approximately 89 L (range: 77 to 133 L) at steady state. There was no detectable plasma protein binding following administration of [14C]-migalastat in the concentration range between 1 to 100 microM.
Elimination
Metabolism
- Based upon in vivo data, migalastat is a substrate for uridine diphosphate glucuronosyltransferase (UDPGT), a minor elimination pathway.
Excretion
- In a mass balance study in healthy male subjects, following oral administration of 123 mg [14C]-migalastat, approximately 77% of the total radiolabeled dose was recovered in urine and 20% of the total radiolabeled dose was recovered in feces with an overall total recovery of 98% within 96 hours post-dose. In urine, unchanged migalastat accounted for 80% of the radioactivity, which equates to 62% of the administered dose. In feces, unchanged migalastat was the only drug-related component. In plasma, unchanged migalastat accounted for approximately 77% of the plasma radioactivity and three dehydrogenated O-glucuronide conjugated metabolites, M1 to M3, together accounted for approximately 13% of the plasma radioactivity, none of which comprised more than 6% of the radiolabeled dose. Approximately 9% of the total radioactivity in plasma was unassigned.
- Following a single oral dose of 123 mg migalastat, migalastat is cleared from plasma with a mean half-life (t½) of approximately 4 hours and apparent clearance of 12.5 L/hr.
Specific Populations
- Male and Female Patients: The pharmacokinetic characteristics of migalastat were not significantly different between healthy male and female subjects or patients with Fabry disease.
- Racial or Ethnic Groups: Clinical data indicate no ethnic differences in patient populations studied with migalastat.
- Patients with Renal Impairment: In a single-dose study in subjects with varying degrees of renal impairment, exposure to migalastat (AUC) was increased by 1.2-, 1.8-, and 4.3-fold in subjects with mild (eGFR 60 to 90 mL/min/1.73 m2), moderate (eGFR 30 to 59 mL/min/1.73 m2), and severe renal impairment (eGFR less than 30 mL/min/1.73 m2), respectively, while the Cmax remained unchanged with severity of renal impairment.
Drug Interaction Studies
- Migalastat is not a known inhibitor or inducer of cytochrome P450 (CYP450) enzymes, nor is it an inhibitor of BCRP, MDR1, P-glycoprotein (P-gp), or BSEP human efflux transporters, or OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2, MATE1, or MATE2-K human uptake transporters. Migalastat is not a substrate of P-gp, BCRP, MDR1 or MATE1, MATE2-K, OAT1, OAT3, or OCT2. Migalastat showed low affinity for SGLT1, as both a substrate and an inhibitor, and showed no activity for SGLT2.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- The carcinogenic potential of migalastat was assessed in a 2-year study in rats and a 26-week study in Tg.rasH2 mice. In the 2-year rat study, migalastat was not tumorigenic at oral doses of up to 600 mg/kg twice daily (24 times the recommended dose based on AUC). In the 26-week study in Tg.rasH2 mice, migalastat was not tumorigenic at oral doses of up to 1000 mg/kg/day in males and 500 mg/kg/day in females.
Mutagenesis
- Migalastat was negative in the bacterial mutagenicity (Ames) assay, in vitro cell mutation assay in L5178Y mouse lymphoma TK+/- cells, and in vivo micronucleus assay in rats.
Impairment of Fertility
- Oral administration of up to 12.5 mg/kg migalastat twice daily in rats (equivalent to the human AUC at the recommended dose) produced a significant decrease in male fertility. This effect was completely reversed after four weeks of recovery. Female fertility was not affected.
Clinical Studies
- Study AT1001-011 (referred to as Study 1; NCT00925301) included a 6-month randomized, double-blind, placebo-controlled phase followed by a 6-month open-label treatment phase and a 12-month open-label extension phase. Patients received the recommended dosage of 123 mg migalastat every other day taken without consuming food 2 hours before and 2 hours after each dose to give a minimum 4 hour fast. A total of 67 patients with Fabry disease who were naïve to migalastat and enzyme replacement therapy (ERT) or were previously treated with ERT (agalsidase beta or non-U.S. approved agalsidase alfa) and had been off ERT for at least 6 months were randomized in a 1:1 ratio to receive either migalastat 123 mg every other day or placebo for the first 6 months. In the second 6 months, all patients were treated with migalastat. Of the 67 enrolled patients, 50 patients (32 females, 18 males) had amenable GLA variants based on the in vitro amenability assay. The median age of the population was 45 years and 97% were Caucasian. The major efficacy outcome measure of the average number of GL-3 inclusions per kidney interstitial capillary (KIC) in renal biopsy samples was assessed by light microscopy before and after treatment. Efficacy was evaluated after 6 months of treatment in 45 of 50 patients (29 females and 16 males) with available histology data both at baseline and month 6. Of the 45 evaluable patients, 25 received migalastat (18 females, 7 males) and 20 received placebo (11 females, 9 males). The proportion of patients with ≥ 50% reduction from baseline in the average number of GL-3 inclusions per KIC and the median changes from baseline in the average number of GL-3 inclusions per KIC after 6 months of treatment in Study 1 are shown in TABLE 3.
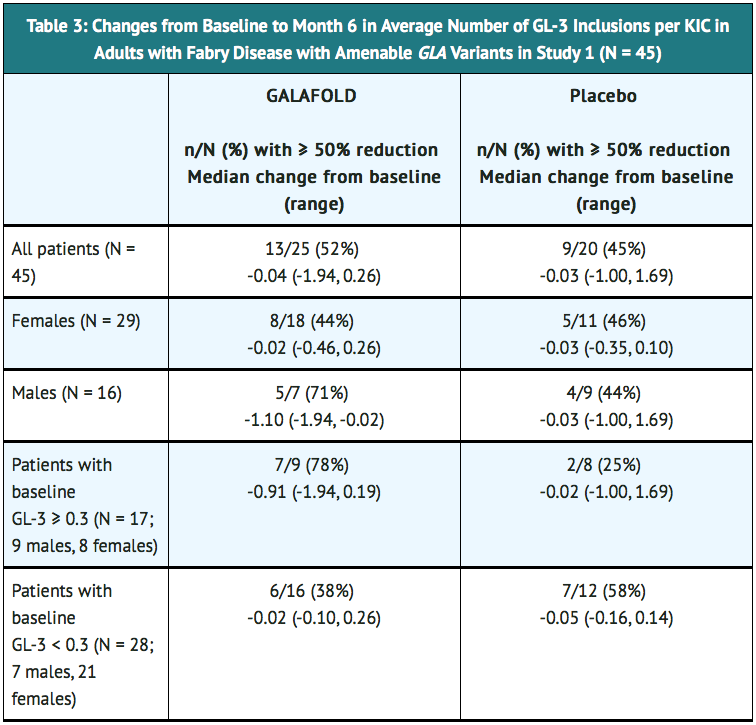
- In Study 1, patients with non-amenable GLA variants (n = 17) had no change from baseline in the average number of GL-3 inclusions per KIC after 6 months of treatment.
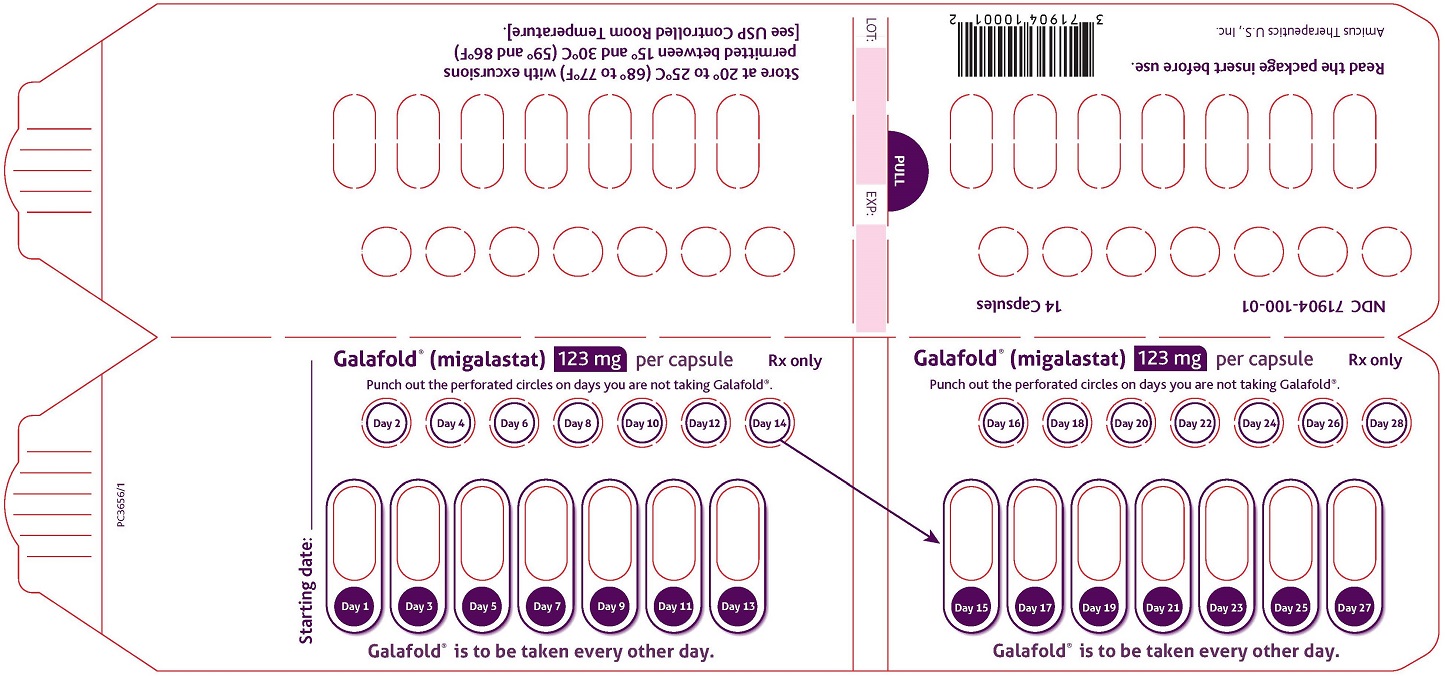
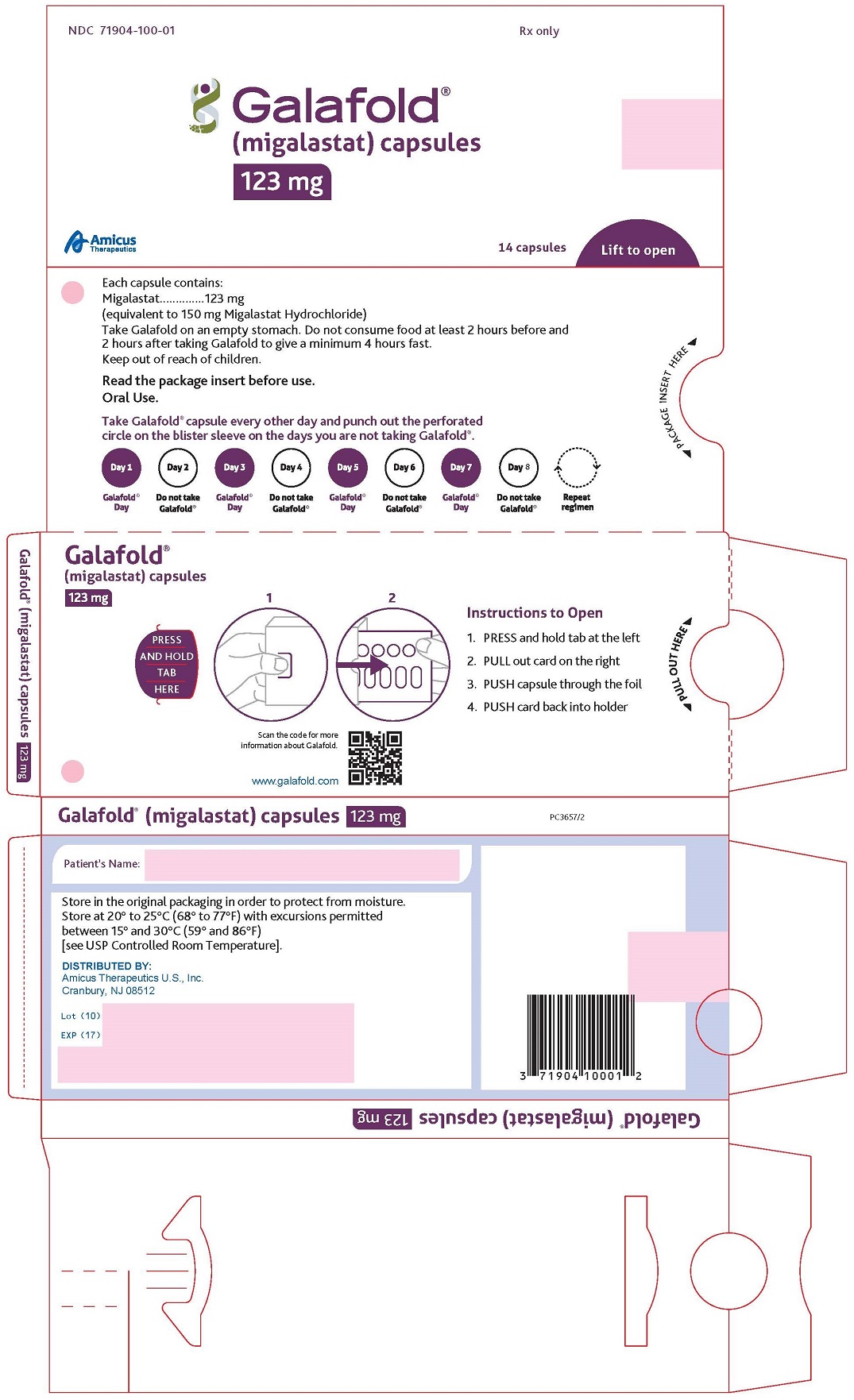
How Supplied
- Migalastat capsules are supplied as 123 mg migalastat, size “2” capsules with opaque blue cap and opaque white body filled with white to pale brown powder and imprinted with “A1001” in black ink.
- Migalastat capsules are packaged as two 7-count capsules blister strips with aluminum foil lidding encased in cardboard blister cards providing 14 capsules per wallet pack that supplies the drug product for 4 weeks (28 days).
- Wallet pack containing 14 migalastat capsules NDC 71904-100-01.
Storage
- Store at USP Controlled Room Temperature of 20° to 25°C (68° to 77°F) with excursions permitted between 15° and 30°C (59° and 86°F).
Images
Drug Images
{{#ask: Page Name::Migalastat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Migalastat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient:
- To take migalastat once every other day at the same time of day.
- Take migalastat on an empty stomach. Do not consume food at least 2 hours before and 2 hours after taking migalastat to give a minimum 4 hours fast. Clear liquids can be consumed during this 4-hour period.
- Not to take migalastat on 2 consecutive days.
- If a dose is missed entirely for the day, take the missed dose only if it is within 12 hours of the normal time that the dose should have been taken. If more than 12 hours have passed, resume taking migalastat at the next planned dosing day and time, according to the every-other-day dosing schedule.
- Swallow capsules whole. Do not cut, crush, or chew.
Patient Package Insert
Instructions for Use
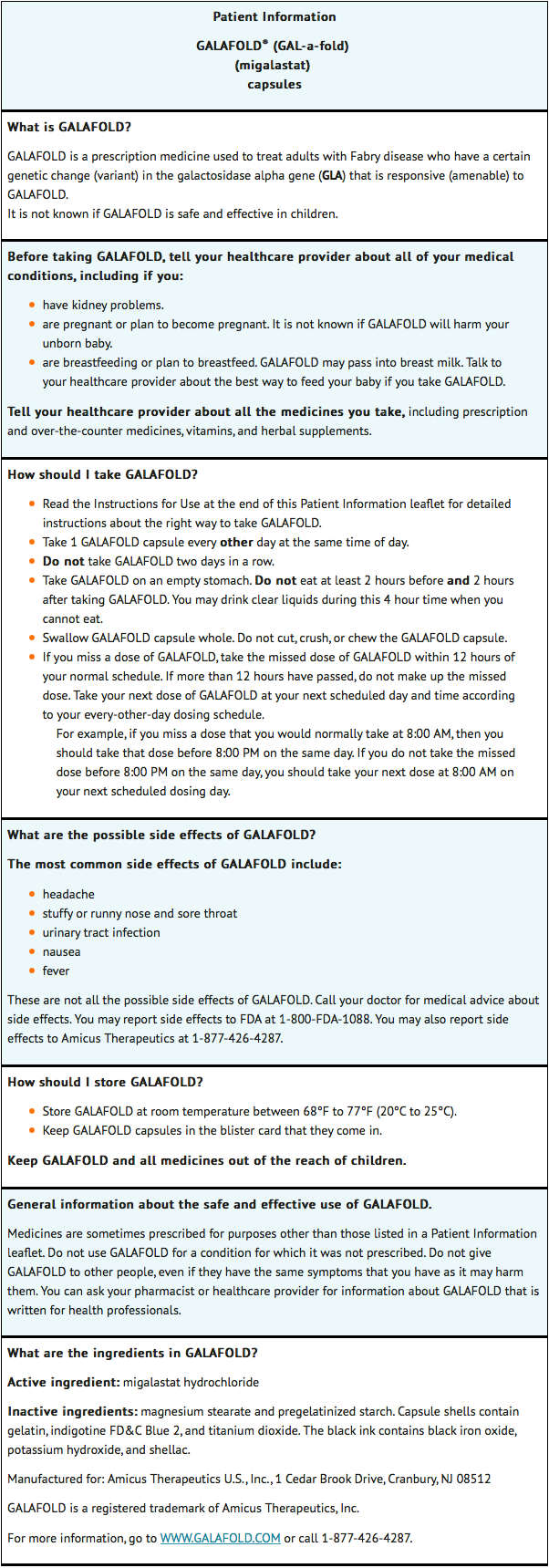
- Read this Instructions for Use before you start taking migalastat and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Important information:
- Take 1 migalastat capsule every other day at the same time of day.
- Do not take migalastat two days in a row.
- Take migalastat on an empty stomach. Do not eat at least 2 hours before and 2 hours after taking migalastat. You may drink clear liquids during this 4 hour time when you cannot eat.
- Swallow the migalastat capsule whole. Do not cut, crush, or chew the migalastat capsule.
Opening the migalastat capsule carton:
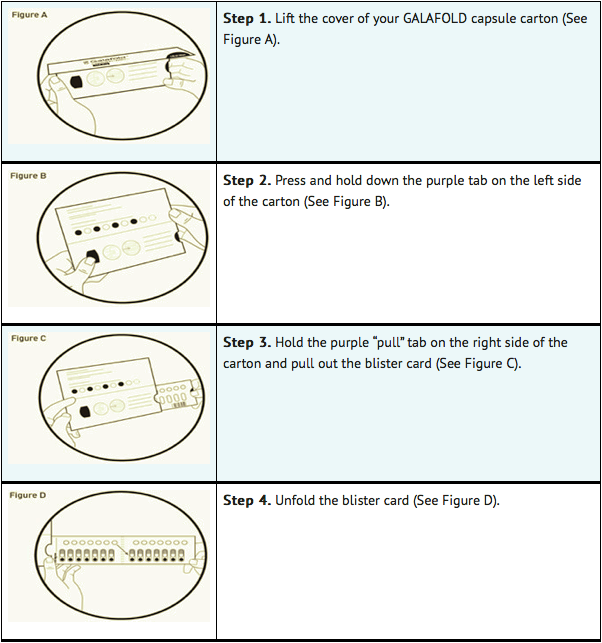
Taking migalastat capsules:
- Each migalastat blister card contains 14 migalastat capsules (enough for 28 days of treatment with migalastat) and 14 white cardboard circles. The white cardboard circles are to remind you to take migalastat every other day (See Figure E).
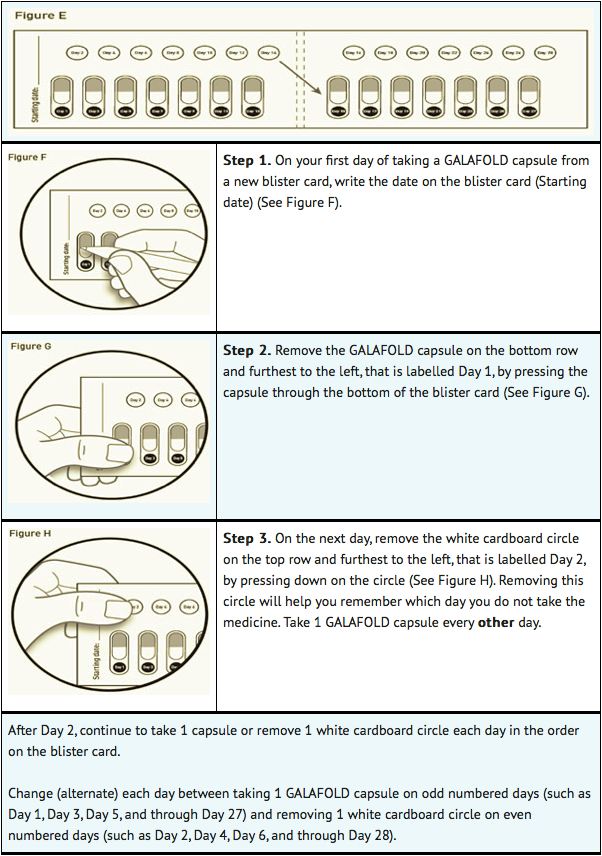
- Replace the blister card back in the carton after each use.
Precautions with Alcohol
Alcohol-Migalastat interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Migalastat Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.