Metronidazole Topical
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Metronidazole Topical is a nitroimidazole that is FDA approved for the {{{indicationType}}} of inflammatory lesions of rosacea. Common adverse reactions include nasopharyngitis, upper respiratory tract infection, and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Rosacea
- Gel, 1%. METROGEL is a clear, colorless to pale yellow gel. Each gram of METROGEL contains 10mg (1%) of metronidazole.
- Apply and rub in a thin film of METROGEL once daily to affected area(s).
- A gentle cleanser should be used before the application of METROGEL.
- Cosmetics may be applied after the application of METROGEL.
- Not for oral, ophthalmic or intravaginal use.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Metronidazole Topical in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Metronidazole Topical in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Metronidazole Topical in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Metronidazole Topical in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Metronidazole Topical in pediatric patients.
Contraindications
- METROGEL is contraindicated in patients with a history of hypersensitivity to metronidazole or to any other ingredient in the formulation.
Warnings
Precautions
- Neurologic Disease
- Peripheral neuropathy, characterized by numbness or paresthesia of an extremity has been reported in patients treated with systemic metronidazole. Although not evident in clinical trials for topical metronidazole, peripheral neuropathy has been reported with the post approval use. The appearance of abnormal neurologic signs should prompt immediate reevaluation of METROGEL therapy. Metronidazole should be administered with caution to patients with central nervous system diseases.
- Blood Dyscrasias
- Metronidazole is a nitroimidazole; use with care in patients with evidence of, or history of, blood dyscrasia.
- Contact Dermatitis
- Irritant and allergic contact dermatitis have been reported. If dermatitis occurs, patients may need to discontinue use.
- Eye Irritation
- Topical metronidazole has been reported to cause tearing of the eyes. Avoid contact with the eyes.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
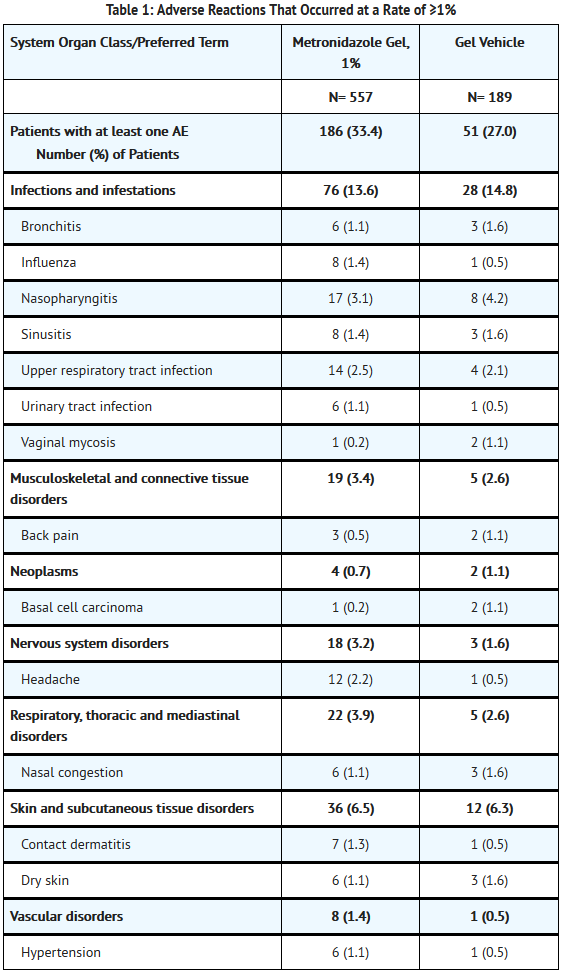
In a controlled clinical trial, 557 patients used metronidazole gel, 1% and 189 patients used the gel vehicle once daily for up to 10 weeks. The following table summarizes selected adverse reactions that occurred at a rate of ≥1%:
T1
T2
- The following additional adverse experiences have been reported with the topical use of metronidazole: skin irritation, transient redness, metallic taste, tingling or numbness of extremities, and nausea.
Postmarketing Experience
- The following adverse reaction has been identified during post approval use of topical metronidazole: peripheral neuropathy. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Drug Interactions
- Oral metronidazole has been reported to potentiate the anticoagulant effect of coumarin and warfarin, resulting in a prolongation of prothrombin time. Drug interactions should be kept in mind when METROGEL is prescribed for patients who are receiving anticoagulant treatment, although they are less likely to occur with topical metronidazole administration because of low absorption.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- There are no adequate and well-controlled studies with the use of METROGEL in pregnant women.
- Metronidazole crosses the placental barrier and enters the fetal circulation rapidly. No fetotoxicity was observed after oral administration of metronidazole in rats or mice at 200 and 20 times, respectively, the expected clinical dose. However, oral metronidazole has shown carcinogenic activity in rodents. Because animal reproduction studies are not always predictive of human response, METROGEL should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Metronidazole Topical in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Metronidazole Topical during labor and delivery.
Nursing Mothers
- After oral administration, metronidazole is secreted in breast milk in concentrations similar to those found in the plasma. Even though blood levels taken after topical metronidazole application are significantly lower than those achieved after oral metronidazole a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother and the risk to the infant.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Sixty-six subjects aged 65 years and older were treated with metronidazole gel, 1% in the clinical study. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Metronidazole Topical with respect to specific gender populations.
Race
There is no FDA guidance on the use of Metronidazole Topical with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Metronidazole Topical in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Metronidazole Topical in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Metronidazole Topical in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Metronidazole Topical in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Metronidazole Topical in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Metronidazole Topical in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- There are no reported human experiences with overdosage of METROGEL. Topically applied metronidazole can be absorbed in sufficient amount to produce systemic effects.
Chronic Overdose
There is limited information regarding Chronic Overdose of Metronidazole Topical in the drug label.
Pharmacology
Mechanism of Action
- The mechanism of action of metronidazole in the treatment of rosacea is unknown.
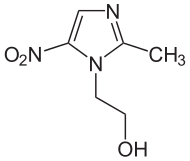
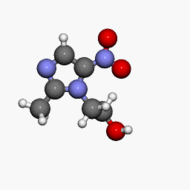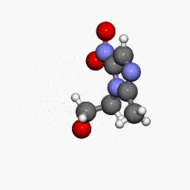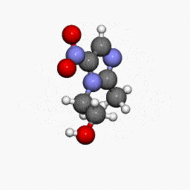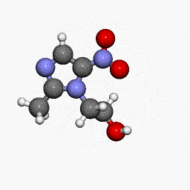
Structure
- METROGEL (metronidazole) Gel, 1% contains metronidazole, USP. Chemically, metronidazole is 2-methyl-5-nitro-1 H-imidazole-1-ethanol. The molecular formula for metronidazole is C6H9N3O3. It has the following structural formula:
- Metronidazole has a molecular weight of 171.16. It is a white to pale yellow crystalline powder. It is slightly soluble in alcohol and has solubility in water of 10 mg/mL at 20˚C. Metronidazole belongs to the nitroimidazole class of compounds.
- METROGEL is a clear, colorless to pale yellow, aqueous gel; each gram contains 10 mg of metronidazole in a base of betadex, edetate disodium, hydroxyethyl cellulose, methylparaben, niacinamide, phenoxyethanol, propylene glycol, propylparaben and purified water.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Metronidazole Topical in the drug label.
Pharmacokinetics
- Topical administration of a one gram dose of METROGEL to the face of 13 patients with moderate to severe rosacea once daily for 7 days resulted in a mean ± SD Cmax of metronidazole of 32 ± 9 ng/mL. The mean ± SD AUC(0-24) was 595 ± 154 ng*hr/mL. The mean Cmax and AUC(0-24) are less than 1% of the value reported for a single 250 mg oral dose of metronidazole. The time to maximum plasma concentration (Tmax) was 6-10 hours after topical application.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Metronidazole Topical in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Metronidazole Topical in the drug label.
How Supplied
Storage
There is limited information regarding Metronidazole Topical Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Metronidazole Topical |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Metronidazole Topical |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Metronidazole Topical in the drug label.
Precautions with Alcohol
- Alcohol-Metronidazole Topical interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[3]
Look-Alike Drug Names
- A® — B®[4]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 1.3 1.4 "Flagyl, Flagyl ER (metronidazole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 3 April 2014.
- ↑ 2.0 2.1 2.2 2.3 2.4 Brayfield, A, ed. (14 January 2014). "Metronidazole". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 3 April 2014.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Metronidazole Topical |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Metronidazole Topical |Label Name=Metronidazole Topical11.png
}}
{{#subobject:
|Label Page=Metronidazole Topical |Label Name=Metronidazole Topical11.png
}}