Methyltestosterone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 20: | Line 20: | ||
|indication= | |indication= | ||
primary hypogonadism, hypogonadotropic hypogonadism, ,delayed puberty, advancing inoperable metastatic (skeletal) mammary cancer | |||
|hasBlackBoxWarning= | |hasBlackBoxWarning= | ||
| Line 43: | Line 43: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
Males—Replacement therapy in conditions associated with deficiency or absence of endogenous testosterone: | |||
Primary hypogonadism (congenital or acquired)—testicular failure due to cryptorchidism, bilateral torsions, orchitis, vanishing testis syndrome; or orchidectomy. | |||
Hypogonadotropic hypogonadism (congenital or acquired)—idiopathic gonadotropin of LHRH deficiency, or pituitary-hypothalamic injury from tumors, trauma, or radiation. If the above conditions occur prior to puberty, androgen replacement therapy will be needed during the adolescent years for development of secondary sexual characteristics. Prolonged androgen treatment will be required to maintain sexual characteristics in these and other males who develop testosterone deficiency after puberty. | |||
Androgens may be used to stimulate puberty in carefully selected males with clearly delayed puberty. These patients usually have a familial pattern of delayed puberty that is not secondary to a pathological disorder; puberty is expected to occur spontaneously at a relatively late date. Brief treatment with conservative doses may occasionally be justified in these patients if they do not respond to psychological support. The potential adverse effect on bone maturation should be discussed with the patient and parents prior to androgen administration. An X-ray of the hand and wrist to determine bone age should be obtained every 6 months to assess the effect of treatment on the epiphyseal centers (see WARNINGS). | |||
Females—Androgens may be used secondarily in women with advancing inoperable metastatic (skeletal) mammary cancer who are 1 to 5 years postmenopausal. Primary goals of therapy in these women include ablation of the ovaries. Other methods of counteracting estrogen activity are adrenalectomy, hypophysectomy, and/or anti-estrogen therapy. This treatment has also been used in premenopausal women with breast cancer who have benefited from oophorectomy and are considered to have a hormone-responsive tumor. Judgment concerning androgen therapy should be made by an oncologist with expertise in this field. | |||
Dosage must be strictly individualized. The suggested dosage for androgens varies depending on the age, sex, and diagnosis of the individual patient. Adjustments and duration of dosage will depend upon the patient's response and the appearance of adverse reactions. | |||
Males | |||
In the androgen-deficient male the guideline for replacement therapy indicates the usual initial dosage of 10-50 mg daily. | |||
Various dosage regimens have been used to induce pubertal changes in hypogonadel males: some experts have advocated lower dosages initially, gradually increasing the dose as puberty progresses, with or without a decrease to maintenance levels. Other experts emphasize that higher dosages are needed to induce pubertal changes and lower dosages can be used for maintenance after puberty. The chronological and skeletal ages must be taken into consideration, both in determining the initial dose and in adjusting the dose. | |||
Dosages used in delayed puberty generally are in the lower ranges of those given above, and are for limited duration, for example, 4 to 6 months. | |||
Females | |||
Women with metastatic breast carcinoma must be followed closely because androgen therapy occasionally appears to accelerate the disease. Thus, many experts prefer to use the shorter acting androgen preparations rather than those with prolonged activity for treating breast carcinoma particularly during the early stages of androgen therapy. | |||
Guideline dosages of androgens for use in the palliative treatment of women with metastatic breast cancer are 50-200 mg daily. | |||
=====Condition1===== | =====Condition1===== | ||
| Line 160: | Line 190: | ||
|contraindications= | |contraindications= | ||
* | *Methyltestosterone Tablets are contraindicated in men with carcinomas of the breast or with known or suspected carcinomas of the prostate, and in women who are or may become pregnant. When administered to pregnant women, androgens cause virilization of the external genitalia of the female fetus. This virilization includes clitoromegaly, abnormal vaginal development, and fusion of genital folds to form a scrotal-like structure. The degree of masculinization is related to the amount of drug given and age of the fetus, and is most likely to occur in the female fetus when the drugs are given in the first trimester. If the patient becomes pregnant while taking these drugs, she should be apprised of the potential hazard to the fetus. | ||
<!--Warnings--> | <!--Warnings--> | ||
| Line 166: | Line 196: | ||
|warnings= | |warnings= | ||
* | *In patients with breast cancer, androgen therapy may cause hypercalcemia by stimulating osteolysis. In this case, the drug should be discontinued. | ||
*Prolonged use of high doses of androgens has been associated with the development of peliosis hepatis and hepatic neoplasms including hepatocellular carcinoma. Peliosis hepatis can be a life-threatening or fatal complication. | |||
*Cholestatic hepatitis and jaundice occur with 17-alpha-alkylandrogens (such as methyltestosterone) at a relatively low dose. If cholestatic hepatitis with jaundice appears or if liver function tests become abnormal, the androgen should be discontinued and the etiology should be determined. Drug-induced jaundice is reversible when the medication is discontinued. | |||
*Geriatric patients treated with androgens may be at an increased risk of developing prostatic hypertrophy and prostatic carcinoma although conclusive evidence to support this concept is lacking. | |||
There | *There have been postmarketing reports of venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), in patients using testosterone products, such as methyltestosterone. Evaluate patients who report symptoms of pain, edema, warmth and erythema in the lower extremity for DVT and those who present with acute shortness of breath for PE. If a venous thromboembolic event is suspected, discontinue treatment with methyltestosterone and initiate appropriate workup and management. | ||
*Edema with or without congestive heart failure may be a serious complication in patients with pre-existing cardiac, renal or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required. | |||
*Gynecomastia frequently develops and occasionally persists in patients being treated for hypogonadism. | |||
*Androgen therapy should be used cautiously in healthy males with delayed puberty. The effect on bone maturation should be monitored by assessing bone age of the wrist and hand every 6 months. In children, androgen treatment may accelerate bone maturation without producing compensatory gain in linear growth. This adverse effect may result in compromised adult stature. The younger the child the greater the risk of compromising final mature height. | |||
*This drug has not been shown to be safe and effective for the enhancement of athletic performance. Because of the potential risk of serious adverse health effects, this drug should not be used for such purpose. | |||
==== | ====Precautions==== | ||
*Woman should be observed for signs of virilization (deepening of the voice, hirsutism, acne, clitoromegaly, and menstrual irregularities). Discontinuation of drug therapy at the time of evidence of mild virilism is necessary to prevent irreversible virilization. Such virilization is usual following androgen use at high doses. The patient and physician may decide that some virilization will be tolerated during treatment for breast carcinoma. | |||
*Priapism or excessive sexual stimulation may develop. Males, especially the elderly, may become overstimulated. In treating males for symptoms of climacteric, avoid stimulation to the point of increasing the nervous, mental, and physical activities beyond the patient's cardiovascular capacity. | |||
*Oligospermia and reduced ejaculatory volume may occur after prolonged administration or excessive dosage. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
=====Endocrine and Urogenital===== | |||
*Female | |||
:*The most common side effects of androgen therapy are amenorrhea and other menstrual irregularities, inhibition of gonadotropin secretion, and virilization, including deepening of the voice and clitoral enlargement. The latter usually is not reversible after androgens are discontinued. When administered to a pregnant woman, androgens cause virilization of external genitalia of the female fetus. | |||
*Male | |||
:*Gynecomastia, and excessive frequency and duration of penile erections. Oligospermia may occur at high dosage (see CLINICAL PHARMACOLOGY). | |||
=====Skin and Appendages===== | |||
Hirsutism, male pattern of baldness, and acne. | |||
===== | =====Fluid and Electrolyte Disturbances===== | ||
Retention of sodium, chloride, water, potassium, calcium, and inorganic phosphates. | |||
=====Gastrointestinal===== | |||
Nausea, cholestatic jaundice, alterations in liver function tests, rarely hepatocellular neoplasma and peliosis hepatis. | |||
===== | =====Hematologic===== | ||
Suppression of clotting factors II, V, VII, and X, bleeding in patients on concomitant anticoagulant therapy, and polycythemia. | |||
=====Nervous System===== | |||
Increased or decreased libido, headache, anxiety, depression, and generalized paresthesia. | |||
===== | =====Metabolic===== | ||
Increased serum cholesterol. | |||
=====Vascular Disorders===== | |||
Venous thromboembolism. | |||
=====Miscellaneous===== | =====Miscellaneous===== | ||
Rarely, anaphylactoid reactions. | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
| Line 249: | Line 273: | ||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
| Line 306: | Line 278: | ||
|drugInteractions= | |drugInteractions= | ||
* | *Anticoagulants | ||
:* | :*C-17 substituted derivatives of testosterone, such as methandrostenolone have been reported to decrease the anticoagulant requirements of patients receiving oral anticoagulants. Patients receiving anticoagulant therapy require close monitoring, especially when androgens are started or stopped. | ||
*Oxyphenbutazone | |||
:*Concurrent administration of oxyphenbutazone and androgens may result in elevated serum levels of oxyphenbutazone. | |||
*Insulin | |||
:*In diabetic patients, metabolic effects of androgens may decrease blood glucose and insulin requirements. | |||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
* '''Pregnancy Category''' | * '''Pregnancy Category X''' | ||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
| Line 323: | Line 302: | ||
|useInNursing= | |useInNursing= | ||
*It is not known whether androgens are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from androgens, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed= | |useInPed= | ||
*Androgen therapy should be used very cautiously in children and only by specialists who are aware of the adverse effects on bone maturation. Skeletal maturation must be monitored every six months by an X-ray of the hand and wrist (See INDICATIONS & USAGE, and WARNINGS). | |||
|useInGeri= | |useInGeri= | ||
| Line 375: | Line 356: | ||
===Acute Overdose=== | ===Acute Overdose=== | ||
*Overdose of medication may be reflected in the occurrence of the signs and symptoms associated with testosterone-anabolic drugs. Nausea and appearance of the early manifestations of edema should be looked for. However, there has been no report of acute overdosage with androgens. | |||
* | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | ||
| Line 399: | Line 372: | ||
|mechAction= | |mechAction= | ||
* | * Endogenous androgens are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis and scrotum; the development of male hair distribution, such as beard, pubic, chest, and axillary hair; laryngeal enlargement, vocal cord thickening; alterations in body musculature and fat distribution. Drugs in this class also cause retention of nitrogen, sodium, potassium, phosphorus, and decreased urinary excretion of calcium. Androgens have been reported to increase protein anabolism and decrease protein catabolism. Nitrogen balance is improved only when there is sufficient intake of calories and protein. | ||
*Androgens are responsible for the growth spurt of adolescence and for the eventual termination of linear growth which is brought about by fusion of the epiphyseal growth centers. In children, exogenous androgens accelerate linear growth rates, but may cause a disproportionate advancement in bone maturation. Use over long periods may result in fusion of the epiphyseal growth centers and termination of growth process. Androgens have been reported to stimulate the production of red blood cells by enhancing the production of erythropoietic stimulating factor. | |||
*During exogenous administration of androgens, endogenous testosterone release is inhibited through feedback inhibition of pituitary luteinizing hormone (LH). With large doses of exogenous androgens, spermatogenesis may also be suppressed through feedback inhibition of pituitary follicle stimulating hormone (FSH). There is a lack of substantial evidence that androgens are effective in fractures, surgery, convalescence, and functional uterine bleeding. | |||
<!--Structure--> | <!--Structure--> | ||
| Line 405: | Line 382: | ||
|structure= | |structure= | ||
* | * METHITEST™ Tablets contain methyltestosterone, USP, a synthetic androgen. Androgens are steroids that develop and maintain primary and secondary male sex characteristics. Methyltestosterone Tablets are to be taken orally. | ||
*Androgens are derivatives of cyclopentanoperhydrophenanthrena. Endogenous androgens are C-19 steroids with a side chain at C-17, and with two angular methyl groups. Testosterone is the primary endogenous androgen. In their active form, all drugs in the class have a 17-beta-hydroxy group. 17-alpha alkylation (methyltestosterone) increases the pharmacologic activity per unit weight compared to testosterone when given orally. | |||
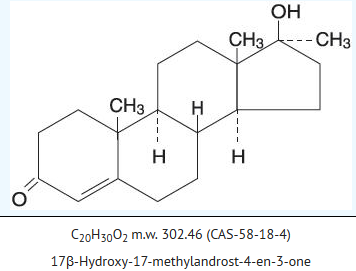
*Methyltestosterone is the 17α-methyl derivative of testosterone, the true testicular hormone. Chemically, methyltestosterone is 17β-hydroxy-17-methylandrost-4-en-3-one, with the empirical formula C20H30O2, a molecular weight of 302.5, and the following structural formula: | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
*Methyltestosterone is a white to creamy-white, odorless, slightly hydroscopic powder. It is practically insoluble in water, and is soluble in alcohol and other organic solvents. Methyltestosterone Tablets contain methyltestosterone, USP and acacia, lactose monohydrate, confectioner's sugar, corn starch, powdered cellulose, sodium lauryl sulfate, magnesium stearate, pregelatinized starch, and guar gum. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 419: | Line 402: | ||
|PK= | |PK= | ||
*Testosterone given orally is metabolized by the gut and 44% is cleared by the liver in the first pass. Oral doses as high as 400 mg per day are needed to achieve clinically effective blood levels for full replacement therapy. The synthetic androgen (methyltestosterone) is less extensively metabolized by the liver and has a longer half-life. It is more suitable than testosterone for oral administration. | |||
*Testosterone in plasma is 98% bound to a specific testosterone-estradiol binding globulin, and about 1% is free. Generally, the amount of this sex-hormone binding globulin in the plasma will determine the distribution of testosterone between free and bound forms, and the free testosterone concentration will determine its half-life. | |||
*About 90% of a dose of testosterone is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver. Testosterone is metabolized to various 17-keto steroids through two different pathways. As reported in the literature, the half-life of testosterone varies considerably, ranging from 10 to 100 minutes. | |||
*In many tissues the activity of testosterone appears to depend on reduction to dihydrotestosterone, which binds to cytosol receptor proteins. The steroid-receptor complex is transported to the nucleus where it initiates transcription events and cellular changes related to androgen action. | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
| Line 425: | Line 414: | ||
|nonClinToxic= | |nonClinToxic= | ||
There is | *Animal Data | ||
:*Testosterone has been tested by subcutaneous injection and implantation in mice and rats. The implant induced cervical-uterine tumors in mice, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats. | |||
*Human Data | |||
:*There are rare reports of hepatocellular carcinoma in patients receiving long-term therapy with androgens in high doses. Withdrawal of the drugs did not lead to regression of the tumors in all cases. | |||
:*Geriatric patients treated with androgens may be at an increased risk of developing prostatic hypertrophy and prostatic carcinoma although conclusive evidence to support this concept is lacking. | |||
:*This compound has not been tested for mutagenic potential. However, as noted above, carcinogenic effects have been attributed to treatment with adrogenic hormones. The potential carcinogenic effects likely occur through a hormonal mechanism rather than by a direct chemical interaction mechanism. | |||
:*Impairment of fertility was not tested directly in animal species. However, as noted below under ADVERSE REACTIONS, oligospermia in males and amenorrhea in females are potential adverse effects of treatment with Methyltestosterone Tablets. Therefore, impairment of fertility is a possible outcome of treatment with Methyltestosterone Tablets. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
| Line 437: | Line 433: | ||
|howSupplied= | |howSupplied= | ||
* | *Each compressed, single-scored, round, white tablet contains 10 mg of methyltestosterone for oral use. Each tablet is debossed with "7037" on one side, and scored on the other side. | ||
:*Bottles of 100 tablets NDC 0115-7037-01 | |||
*Protect from Light, Moisture and Heat. Store at controlled room temperature, 15°-30°C (59°-86°F). | |||
*This package is not for household dispensing. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure, as required. | |||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
| Line 443: | Line 444: | ||
|fdaPatientInfo= | |fdaPatientInfo= | ||
*The physician should instruct patients to report any of the following side effects of androgens: | |||
*Adult or Adolescent Males: Too frequent or persistent erections of the penis. | |||
*Women: Hoarseness, acne, changes in menstrual periods, or more hair on the face. | |||
*All Patients: Any nausea, vomiting, changes in skin color, or ankle swelling. | |||
*Any male adolescent patient receiving androgens for delayed puberty should have bone development checked every 6 months. | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
Revision as of 19:35, 3 November 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Methyltestosterone is a synthetic androgen that is FDA approved for the {{{indicationType}}} of primary hypogonadism, hypogonadotropic hypogonadism, ,delayed puberty, advancing inoperable metastatic (skeletal) mammary cancer. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Males—Replacement therapy in conditions associated with deficiency or absence of endogenous testosterone:
Primary hypogonadism (congenital or acquired)—testicular failure due to cryptorchidism, bilateral torsions, orchitis, vanishing testis syndrome; or orchidectomy.
Hypogonadotropic hypogonadism (congenital or acquired)—idiopathic gonadotropin of LHRH deficiency, or pituitary-hypothalamic injury from tumors, trauma, or radiation. If the above conditions occur prior to puberty, androgen replacement therapy will be needed during the adolescent years for development of secondary sexual characteristics. Prolonged androgen treatment will be required to maintain sexual characteristics in these and other males who develop testosterone deficiency after puberty.
Androgens may be used to stimulate puberty in carefully selected males with clearly delayed puberty. These patients usually have a familial pattern of delayed puberty that is not secondary to a pathological disorder; puberty is expected to occur spontaneously at a relatively late date. Brief treatment with conservative doses may occasionally be justified in these patients if they do not respond to psychological support. The potential adverse effect on bone maturation should be discussed with the patient and parents prior to androgen administration. An X-ray of the hand and wrist to determine bone age should be obtained every 6 months to assess the effect of treatment on the epiphyseal centers (see WARNINGS).
Females—Androgens may be used secondarily in women with advancing inoperable metastatic (skeletal) mammary cancer who are 1 to 5 years postmenopausal. Primary goals of therapy in these women include ablation of the ovaries. Other methods of counteracting estrogen activity are adrenalectomy, hypophysectomy, and/or anti-estrogen therapy. This treatment has also been used in premenopausal women with breast cancer who have benefited from oophorectomy and are considered to have a hormone-responsive tumor. Judgment concerning androgen therapy should be made by an oncologist with expertise in this field.
Dosage must be strictly individualized. The suggested dosage for androgens varies depending on the age, sex, and diagnosis of the individual patient. Adjustments and duration of dosage will depend upon the patient's response and the appearance of adverse reactions.
Males
In the androgen-deficient male the guideline for replacement therapy indicates the usual initial dosage of 10-50 mg daily.
Various dosage regimens have been used to induce pubertal changes in hypogonadel males: some experts have advocated lower dosages initially, gradually increasing the dose as puberty progresses, with or without a decrease to maintenance levels. Other experts emphasize that higher dosages are needed to induce pubertal changes and lower dosages can be used for maintenance after puberty. The chronological and skeletal ages must be taken into consideration, both in determining the initial dose and in adjusting the dose.
Dosages used in delayed puberty generally are in the lower ranges of those given above, and are for limited duration, for example, 4 to 6 months.
Females
Women with metastatic breast carcinoma must be followed closely because androgen therapy occasionally appears to accelerate the disease. Thus, many experts prefer to use the shorter acting androgen preparations rather than those with prolonged activity for treating breast carcinoma particularly during the early stages of androgen therapy.
Guideline dosages of androgens for use in the palliative treatment of women with metastatic breast cancer are 50-200 mg daily.
Condition1
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Methyltestosterone in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methyltestosterone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Methyltestosterone in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Methyltestosterone in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methyltestosterone in pediatric patients.
Contraindications
- Methyltestosterone Tablets are contraindicated in men with carcinomas of the breast or with known or suspected carcinomas of the prostate, and in women who are or may become pregnant. When administered to pregnant women, androgens cause virilization of the external genitalia of the female fetus. This virilization includes clitoromegaly, abnormal vaginal development, and fusion of genital folds to form a scrotal-like structure. The degree of masculinization is related to the amount of drug given and age of the fetus, and is most likely to occur in the female fetus when the drugs are given in the first trimester. If the patient becomes pregnant while taking these drugs, she should be apprised of the potential hazard to the fetus.
Warnings
- In patients with breast cancer, androgen therapy may cause hypercalcemia by stimulating osteolysis. In this case, the drug should be discontinued.
- Prolonged use of high doses of androgens has been associated with the development of peliosis hepatis and hepatic neoplasms including hepatocellular carcinoma. Peliosis hepatis can be a life-threatening or fatal complication.
- Cholestatic hepatitis and jaundice occur with 17-alpha-alkylandrogens (such as methyltestosterone) at a relatively low dose. If cholestatic hepatitis with jaundice appears or if liver function tests become abnormal, the androgen should be discontinued and the etiology should be determined. Drug-induced jaundice is reversible when the medication is discontinued.
- Geriatric patients treated with androgens may be at an increased risk of developing prostatic hypertrophy and prostatic carcinoma although conclusive evidence to support this concept is lacking.
- There have been postmarketing reports of venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), in patients using testosterone products, such as methyltestosterone. Evaluate patients who report symptoms of pain, edema, warmth and erythema in the lower extremity for DVT and those who present with acute shortness of breath for PE. If a venous thromboembolic event is suspected, discontinue treatment with methyltestosterone and initiate appropriate workup and management.
- Edema with or without congestive heart failure may be a serious complication in patients with pre-existing cardiac, renal or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required.
- Gynecomastia frequently develops and occasionally persists in patients being treated for hypogonadism.
- Androgen therapy should be used cautiously in healthy males with delayed puberty. The effect on bone maturation should be monitored by assessing bone age of the wrist and hand every 6 months. In children, androgen treatment may accelerate bone maturation without producing compensatory gain in linear growth. This adverse effect may result in compromised adult stature. The younger the child the greater the risk of compromising final mature height.
- This drug has not been shown to be safe and effective for the enhancement of athletic performance. Because of the potential risk of serious adverse health effects, this drug should not be used for such purpose.
Precautions
- Woman should be observed for signs of virilization (deepening of the voice, hirsutism, acne, clitoromegaly, and menstrual irregularities). Discontinuation of drug therapy at the time of evidence of mild virilism is necessary to prevent irreversible virilization. Such virilization is usual following androgen use at high doses. The patient and physician may decide that some virilization will be tolerated during treatment for breast carcinoma.
- Priapism or excessive sexual stimulation may develop. Males, especially the elderly, may become overstimulated. In treating males for symptoms of climacteric, avoid stimulation to the point of increasing the nervous, mental, and physical activities beyond the patient's cardiovascular capacity.
- Oligospermia and reduced ejaculatory volume may occur after prolonged administration or excessive dosage.
Adverse Reactions
Clinical Trials Experience
Endocrine and Urogenital
- Female
- The most common side effects of androgen therapy are amenorrhea and other menstrual irregularities, inhibition of gonadotropin secretion, and virilization, including deepening of the voice and clitoral enlargement. The latter usually is not reversible after androgens are discontinued. When administered to a pregnant woman, androgens cause virilization of external genitalia of the female fetus.
- Male
- Gynecomastia, and excessive frequency and duration of penile erections. Oligospermia may occur at high dosage (see CLINICAL PHARMACOLOGY).
Skin and Appendages
Hirsutism, male pattern of baldness, and acne.
Fluid and Electrolyte Disturbances
Retention of sodium, chloride, water, potassium, calcium, and inorganic phosphates.
Gastrointestinal
Nausea, cholestatic jaundice, alterations in liver function tests, rarely hepatocellular neoplasma and peliosis hepatis.
Hematologic
Suppression of clotting factors II, V, VII, and X, bleeding in patients on concomitant anticoagulant therapy, and polycythemia.
Nervous System
Increased or decreased libido, headache, anxiety, depression, and generalized paresthesia.
Metabolic
Increased serum cholesterol.
Vascular Disorders
Venous thromboembolism.
Miscellaneous
Rarely, anaphylactoid reactions.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Methyltestosterone in the drug label.
Drug Interactions
- Anticoagulants
- C-17 substituted derivatives of testosterone, such as methandrostenolone have been reported to decrease the anticoagulant requirements of patients receiving oral anticoagulants. Patients receiving anticoagulant therapy require close monitoring, especially when androgens are started or stopped.
- Oxyphenbutazone
- Concurrent administration of oxyphenbutazone and androgens may result in elevated serum levels of oxyphenbutazone.
- Insulin
- In diabetic patients, metabolic effects of androgens may decrease blood glucose and insulin requirements.
Use in Specific Populations
Pregnancy
- Pregnancy Category X
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Methyltestosterone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Methyltestosterone during labor and delivery.
Nursing Mothers
- It is not known whether androgens are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from androgens, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Androgen therapy should be used very cautiously in children and only by specialists who are aware of the adverse effects on bone maturation. Skeletal maturation must be monitored every six months by an X-ray of the hand and wrist (See INDICATIONS & USAGE, and WARNINGS).
Geriatic Use
There is no FDA guidance on the use of Methyltestosterone with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Methyltestosterone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Methyltestosterone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Methyltestosterone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Methyltestosterone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Methyltestosterone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Methyltestosterone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Methyltestosterone in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Methyltestosterone in the drug label.
Overdosage
Acute Overdose
- Overdose of medication may be reflected in the occurrence of the signs and symptoms associated with testosterone-anabolic drugs. Nausea and appearance of the early manifestations of edema should be looked for. However, there has been no report of acute overdosage with androgens.
There is limited information regarding Chronic Overdose of Methyltestosterone in the drug label.
Pharmacology
There is limited information regarding Methyltestosterone Pharmacology in the drug label.
Mechanism of Action
- Endogenous androgens are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis and scrotum; the development of male hair distribution, such as beard, pubic, chest, and axillary hair; laryngeal enlargement, vocal cord thickening; alterations in body musculature and fat distribution. Drugs in this class also cause retention of nitrogen, sodium, potassium, phosphorus, and decreased urinary excretion of calcium. Androgens have been reported to increase protein anabolism and decrease protein catabolism. Nitrogen balance is improved only when there is sufficient intake of calories and protein.
- Androgens are responsible for the growth spurt of adolescence and for the eventual termination of linear growth which is brought about by fusion of the epiphyseal growth centers. In children, exogenous androgens accelerate linear growth rates, but may cause a disproportionate advancement in bone maturation. Use over long periods may result in fusion of the epiphyseal growth centers and termination of growth process. Androgens have been reported to stimulate the production of red blood cells by enhancing the production of erythropoietic stimulating factor.
- During exogenous administration of androgens, endogenous testosterone release is inhibited through feedback inhibition of pituitary luteinizing hormone (LH). With large doses of exogenous androgens, spermatogenesis may also be suppressed through feedback inhibition of pituitary follicle stimulating hormone (FSH). There is a lack of substantial evidence that androgens are effective in fractures, surgery, convalescence, and functional uterine bleeding.
Structure
- METHITEST™ Tablets contain methyltestosterone, USP, a synthetic androgen. Androgens are steroids that develop and maintain primary and secondary male sex characteristics. Methyltestosterone Tablets are to be taken orally.
- Androgens are derivatives of cyclopentanoperhydrophenanthrena. Endogenous androgens are C-19 steroids with a side chain at C-17, and with two angular methyl groups. Testosterone is the primary endogenous androgen. In their active form, all drugs in the class have a 17-beta-hydroxy group. 17-alpha alkylation (methyltestosterone) increases the pharmacologic activity per unit weight compared to testosterone when given orally.
- Methyltestosterone is the 17α-methyl derivative of testosterone, the true testicular hormone. Chemically, methyltestosterone is 17β-hydroxy-17-methylandrost-4-en-3-one, with the empirical formula C20H30O2, a molecular weight of 302.5, and the following structural formula:
- Methyltestosterone is a white to creamy-white, odorless, slightly hydroscopic powder. It is practically insoluble in water, and is soluble in alcohol and other organic solvents. Methyltestosterone Tablets contain methyltestosterone, USP and acacia, lactose monohydrate, confectioner's sugar, corn starch, powdered cellulose, sodium lauryl sulfate, magnesium stearate, pregelatinized starch, and guar gum.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Methyltestosterone in the drug label.
Pharmacokinetics
- Testosterone given orally is metabolized by the gut and 44% is cleared by the liver in the first pass. Oral doses as high as 400 mg per day are needed to achieve clinically effective blood levels for full replacement therapy. The synthetic androgen (methyltestosterone) is less extensively metabolized by the liver and has a longer half-life. It is more suitable than testosterone for oral administration.
- Testosterone in plasma is 98% bound to a specific testosterone-estradiol binding globulin, and about 1% is free. Generally, the amount of this sex-hormone binding globulin in the plasma will determine the distribution of testosterone between free and bound forms, and the free testosterone concentration will determine its half-life.
- About 90% of a dose of testosterone is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver. Testosterone is metabolized to various 17-keto steroids through two different pathways. As reported in the literature, the half-life of testosterone varies considerably, ranging from 10 to 100 minutes.
- In many tissues the activity of testosterone appears to depend on reduction to dihydrotestosterone, which binds to cytosol receptor proteins. The steroid-receptor complex is transported to the nucleus where it initiates transcription events and cellular changes related to androgen action.
Nonclinical Toxicology
- Animal Data
- Testosterone has been tested by subcutaneous injection and implantation in mice and rats. The implant induced cervical-uterine tumors in mice, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
- Human Data
- There are rare reports of hepatocellular carcinoma in patients receiving long-term therapy with androgens in high doses. Withdrawal of the drugs did not lead to regression of the tumors in all cases.
- Geriatric patients treated with androgens may be at an increased risk of developing prostatic hypertrophy and prostatic carcinoma although conclusive evidence to support this concept is lacking.
- This compound has not been tested for mutagenic potential. However, as noted above, carcinogenic effects have been attributed to treatment with adrogenic hormones. The potential carcinogenic effects likely occur through a hormonal mechanism rather than by a direct chemical interaction mechanism.
- Impairment of fertility was not tested directly in animal species. However, as noted below under ADVERSE REACTIONS, oligospermia in males and amenorrhea in females are potential adverse effects of treatment with Methyltestosterone Tablets. Therefore, impairment of fertility is a possible outcome of treatment with Methyltestosterone Tablets.
Clinical Studies
There is limited information regarding Clinical Studies of Methyltestosterone in the drug label.
How Supplied
- Each compressed, single-scored, round, white tablet contains 10 mg of methyltestosterone for oral use. Each tablet is debossed with "7037" on one side, and scored on the other side.
- Bottles of 100 tablets NDC 0115-7037-01
- Protect from Light, Moisture and Heat. Store at controlled room temperature, 15°-30°C (59°-86°F).
- This package is not for household dispensing. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure, as required.
Storage
There is limited information regarding Methyltestosterone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Methyltestosterone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Methyltestosterone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- The physician should instruct patients to report any of the following side effects of androgens:
- Adult or Adolescent Males: Too frequent or persistent erections of the penis.
- Women: Hoarseness, acne, changes in menstrual periods, or more hair on the face.
- All Patients: Any nausea, vomiting, changes in skin color, or ankle swelling.
- Any male adolescent patient receiving androgens for delayed puberty should have bone development checked every 6 months.
Precautions with Alcohol
- Alcohol-Methyltestosterone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Methyltestosterone |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Methyltestosterone |Label Name=Methyltestosterone11.png
}}
{{#subobject:
|Label Page=Methyltestosterone |Label Name=Methyltestosterone11.png
}}