Methazolamide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Methazolamide is a carbonic anhydrase inhibitor that is FDA approved for the {{{indicationType}}} of ocular conditions where lowering intraocular pressure is likely to be of therapeutic benefit, such as chronic open-angle glaucoma, secondary glaucoma, and preoperatively in acute angle-closure glaucoma where lowering the intraocular pressure is desired before surgery. Common adverse reactions include diarrhea, taste alterations, loss of appetite, nausea, vomiting, confusion, paresthesia, somnolence, polyuria, fatigue, and malaise.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Glaucoma
- Dosing Information
- 50–100 mg two or three times daily
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methazolamide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methazolamide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- The safety and effectiveness of methazolamide in children have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methazolamide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methazolamide in pediatric patients.
Contraindications
- Methazolamide therapy is contraindicated in situations in which sodium and/or potassium serum levels are depressed, in cases of marked kidney or liver disease or dysfunction, in adrenal gland failure, and in hyperchloremic acidosis.
- In patients with cirrhosis, use may precipitate the development of hepatic encephalopathy.
- Long-term administration of methazolamide is contraindicated in patients with angle-closure glaucoma, since organic closure of the angle may occur in spite of lowered intraocular pressure.
Warnings
- Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Hypersensitivity reactions may recur when a sulfonamide is readministered, irrespective of the route of administration.
- If hypersensitivity or other serious reactions occur, the use of this drug should be discontinued.
- Caution is advised for patients receiving high-dose aspirin and methazolamide concomitantly, as anorexia, tachypnea, lethargy, coma, and death have been reported with concomitant use of high-dose aspirin and carbonic anhydrase inhibitors.
Precautions
- Potassium excretion is increased initially upon administration of methazolamide and in patients with cirrhosis or hepatic insufficiency could precipitate a hepatic coma.
- In patients with pulmonary obstruction or emphysema, where alveolar ventilation may be impaired, methazolamide should be used with caution because it may precipitate or aggravate acidosis.
Laboratory Tests
- To monitor for hematologic reactions common to all sulfonamides, it is recommended that a baseline CBC and platelet count be obtained on patients prior to initiating methazolamide therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important. Periodic monitoring of serum electrolytes is also recommended.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Methazolamide in the drug label.
Postmarketing Experience
- Adverse reactions, occurring most often early in therapy, include paresthesias, particularly a “tingling” feeling in the extremities; hearing dysfunction or tinnitus; fatigue; malaise; loss of appetite; taste alteration; gastrointestinal disturbances such as nausea, vomiting, and diarrhea; polyuria; and occasional instances of drowsiness and confusion.
- Metabolic acidosis and electrolyte imbalance may occur.
- Transient myopia has been reported. This condition invariably subsides upon diminution or discontinuance of the medication.
- Other occasional adverse reactions include urticaria, melena, hematuria, glycosuria, hepatic insufficiency, flaccid paralysis, photosensitivity, convulsions, and, rarely, crystalluria and renal calculi.
- Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias.
Drug Interactions
- Methazolamide should be used with caution in patients on steroid therapy because of the potential for developing hypokalemia.
- Caution is advised for patients receiving high-dose aspirin and methazolamide concomitantly, as anorexia, tachypnea, lethargy, coma and death have been reported with concomitant use of high-dose aspirin and carbonic anhydrase inhibitors.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Methazolamide has been shown to be teratogenic (skeletal anomalies) in rats when given in doses approximately 40 times the human dose. There are no adequate and well controlled studies in pregnant women. Methazolamide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Methazolamide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Methazolamide during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from methazolamide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of methazolamide in children have not been established.
Geriatic Use
There is no FDA guidance on the use of Methazolamide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Methazolamide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Methazolamide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Methazolamide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Methazolamide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Methazolamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Methazolamide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Methazolamide in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Methazolamide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Methazolamide in the drug label.
Pharmacology
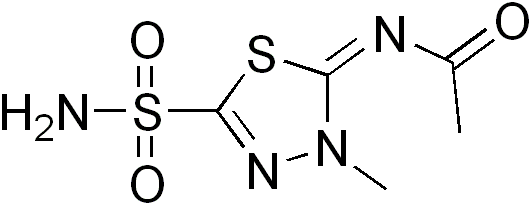
| |
Methazolamide
| |
| Systematic (IUPAC) name | |
| N-(3-methyl-5-sulfamoyl-3H- 1,3,4-thiadiazol-2-ylidene) ethanamide | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 236.274 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 55% |
| Metabolism | ? |
| Half life | 14 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Methazolamide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Methazolamide in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Methazolamide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Methazolamide in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Methazolamide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Methazolamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Methazolamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Methazolamide in the drug label.
Precautions with Alcohol
- Alcohol-Methazolamide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Neptazane®
- Glauctabs®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Methazolamide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Methazolamide |Label Name=Methazolamide11.png
}}
{{#subobject:
|Label Page=Methazolamide |Label Name=Methazolamide11.png
}}
