Mepenzolate: Difference between revisions
Adeel Jamil (talk | contribs) No edit summary |
Adeel Jamil (talk | contribs) No edit summary |
||
| Line 5: | Line 5: | ||
|drugClass=anticholinergic and antispasmodic agent | |drugClass=anticholinergic and antispasmodic agent | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication= peptic ulcer | |indication=peptic ulcer | ||
|adverseReactions=somnolence and blurred vision | |adverseReactions=somnolence and blurred vision | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
| Line 98: | Line 96: | ||
* CANTIL is contraindicated in intestinal atony of the elderly. CANTIL should be used with caution in the elderly. | * CANTIL is contraindicated in intestinal atony of the elderly. CANTIL should be used with caution in the elderly. | ||
|administration=* Oral | |||
|overdose======Signs and Symptoms===== | |overdose======Signs and Symptoms===== | ||
| Line 117: | Line 116: | ||
* Treatment should consist of gastric lavage, emetics, and activated charcoal. Sedatives (e.g., short-acting barbiturates, benzodiazepines) may be used for management of overt signs of excitement. If indicated, an appropriate parenteral cholinergic agent may be used as an antidote. | * Treatment should consist of gastric lavage, emetics, and activated charcoal. Sedatives (e.g., short-acting barbiturates, benzodiazepines) may be used for management of overt signs of excitement. If indicated, an appropriate parenteral cholinergic agent may be used as an antidote. | ||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 381524483 | |||
| IUPAC_name = 3-(2-hydroxy-2,2-diphenylacetoxy)-1,1-dimethylpiperidinium | |||
| image = Mepenzolate.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|CDI|mepenzolate}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 25990-43-6 | |||
| ATC_prefix = A03 | |||
| ATC_suffix = AB12 | |||
| PubChem = 4057 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ChemSpiderID = 3917 | |||
| UNII_Ref = {{fdacite|changed|FDA}} | |||
| UNII = ONW3LB39P7 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 524004 | |||
<!--Chemical data--> | |||
| C=21 | H=26 | N=1 | O=3 | charge = + | |||
| molecular_weight = 340.44 g/mol | |||
}} | |||
|mechAction=* CANTIL diminishes gastric acid and pepsin secretion. CANTIL also suppresses spontaneous contractions of the colon. Pharmacologically, it is a post-ganglionic parasympathetic inhibitor. | |mechAction=* CANTIL diminishes gastric acid and pepsin secretion. CANTIL also suppresses spontaneous contractions of the colon. Pharmacologically, it is a post-ganglionic parasympathetic inhibitor. | ||
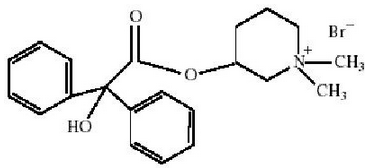
|structure=* CANTIL tablets for oral administration contain 25 mg mepenzolate bromide USP. The anticholinergic agent mepenzolate bromide USP chemically is 3-[(hydroxydiphenylacetyl)oxy]-1,1-dimethylpiperidinium bromide and has the following structure: | |structure=* CANTIL tablets for oral administration contain 25 mg mepenzolate bromide USP. The anticholinergic agent mepenzolate bromide USP chemically is 3-[(hydroxydiphenylacetyl)oxy]-1,1-dimethylpiperidinium bromide and has the following structure: | ||
[[File:Mepenzolate.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|PK=* Radiotracer studies in which CANTIL-14C was used in animals and humans indicate the absorption following oral administration, as with other quaternary ammonium compounds, is low. Between 3 and 22% of an orally administered dose is excreted in the urine over a 5-day period, with the majority of the radioactivity appearing on Day 1. The remainder appears in the next 5 days in the feces and presumably has not been absorbed. | |PK=* Radiotracer studies in which CANTIL-14C was used in animals and humans indicate the absorption following oral administration, as with other quaternary ammonium compounds, is low. Between 3 and 22% of an orally administered dose is excreted in the urine over a 5-day period, with the majority of the radioactivity appearing on Day 1. The remainder appears in the next 5 days in the feces and presumably has not been absorbed. | ||
|howSupplied=25 mg mepenzolate bromide, compressed yellow tablets debossed MERRELL 37. | |howSupplied=25 mg mepenzolate bromide, compressed yellow tablets debossed MERRELL 37. | ||
| Line 137: | Line 185: | ||
sanofi aventis | sanofi aventis | ||
[[File:Mepenzolate drg lael01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo=* CANTIL may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness, such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug. | |fdaPatientInfo=* CANTIL may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness, such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug. | ||
|alcohol=Alcohol-Mepenzolate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Mepenzolate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* Cantil | |brandNames=* Cantil | ||
}} | }} | ||
Revision as of 01:39, 1 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Mepenzolate is an anticholinergic and antispasmodic agent that is FDA approved for the treatment of peptic ulcer. Common adverse reactions include somnolence and blurred vision.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- CANTIL is indicated for use as adjunctive therapy in the treatment of peptic ulcer. It has not been shown to be effective in contributing to the healing of peptic ulcer, decreasing the rate of recurrence, or preventing complications.
Dosing Information
- The usual adult dose is 1 or 2 tablets (25 or 50 mg) 4 times a day preferably with meals and at bedtime. Begin with the lower dosage when possible and adjust subsequently according to the patient's response.
- Safety and efficacy in pediatric patients have not been established.
- In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mepenzolate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mepenzolate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Mepenzolate FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mepenzolate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mepenzolate in pediatric patients.
Contraindications
- Glaucoma
- Obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy)
- Obstructive disease of the gastrointestinal tract (for example, pyloroduodenal stenosis, achalasia)
- Paralytic ileus
- Intestinal atony of the elderly or debilitated patient
- Unstable cardiovascular status in acute gastrointestinal hemorrhage
- Toxic megacolon complicating ulcerative colitis
- Myasthenia gravis
- Allergic or idiosyncratic reactions to CANTIL or related compounds
Warnings
- In the presence of high environmental temperature, heat prostration (fever and heat stroke due to decreased sweating) can occur with use of CANTIL.
- Diarrhea may be an early symptom of incomplete intestinal obstruction especially in patients with ileostomy or colostomy. In this instance, treatment with this drug would be inappropriate and possibly harmful.
- CANTIL may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug.
- With overdosage, a curare-like action may occur i.e., neuromuscular blockage leading to muscular weakness and possible paralysis.
- It should be noted that the use of anticholinergic drugs in the treatment of gastric ulcer may produce a delay in gastric emptying time and may complicate such therapy (antral stasis).
- Psychosis has been reported in sensitive individuals given anticholinergic drugs. CNS signs and symptoms include confusion, disorientation, short-term memory loss, hallucinations, dysarthria, ataxia, coma, euphoria, decreased anxiety, fatigue, insomnia, agitation and mannerisms and inappropriate affect. These CNS signs and symptoms usually resolve within 12 to 24 hours after discontinuation of the medication.
General
- Use CANTIL with caution in the elderly (see PRECAUTIONS, GERIATRIC USE) and in all patients with:
- Autonomic neuropathy
- Hepatic or renal disease
- Ulcerative colitis. Large doses may suppress intestinal motility to the point of producing a paralytic ileus and for this reason precipitate or aggravate "toxic megacolon," a serious complication of the disease.
- Hiatal hernia associated with reflux esophagitis, since anticholinergic drugs may aggravate this condition.
- Coronary heart disease
- Congestive heart failure
- Cardiac arrhythmias
- Tachycardia
- Hypertension
- Prostatic hypertrophy
- Hyperthyroidism
- Investigate any tachycardia before giving anticholinergic (atropine-like) drugs since they may increase the heart rate.
- This product contains FD&C Yellow No. 5 (tartrazine), which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin sensitivity.
Adverse Reactions
Clinical Trials Experience
- Precise frequency data from controlled clinical studies with CANTIL are not available.
Gastrointestinal System:
- Vomiting, nausea, constipation, loss of taste, bloated feeling, dry mouth
Central Nervous System:
- Mental confusion, dizziness, weakness, drowsiness, headache, nervousness
Ophthalmologic:
- Increased ocular tension, cycloplegia, blurred vision, dilation of the pupil
Dermatologic-Hypersensitivity:
- Anaphylaxis, urticaria
Cardiovascular:
- Tachycardia, palpitations
Genitourinary:
- Urinary retention, urinary hesitancy
Miscellaneous:
- Decreased sweating, drowsiness, insomnia, impotence, suppression of lactation
Postmarketing Experience
There is limited information regarding Mepenzolate Postmarketing Experience in the drug label.
Drug Interactions
- The following agents may increase certain actions or side effects of anticholinergic drugs: amantadine, antiarrhythmic agents of class I (e.g., quinidine), antihistamines, antipsychotic agents (e.g., phenothiazines), benzodiazepines, MAO inhibitors, narcotic analgesics (e.g., meperidine), nitrates and nitrites, sympathomimetic agents, tricyclic antidepressants, and other drugs having anticholinergic activity.
- Anticholinergics antagonize the effects of antiglaucoma agents. Anticholinergic drugs in the presence of increased intraocular pressure may be hazardous when taken concurrently with agents such as corticosteroids.
- Anticholinergic agents may affect gastrointestinal absorption of various drugs, such as slowly dissolving dosage forms of digoxin; increased serum digoxin concentrations may result. Anticholinergic drugs may antagonize the effects of drugs that alter gastrointestinal motility, such as metoclopramide. Because antacids may interfere with the absorption of anticholinergic agents, simultaneous use of these drugs should be avoided.
- The inhibiting effects of anticholinergic drugs on gastric hydrochloric acid secretion are antagonized by agents used to treat achlorhydria and those used to test gastric secretion.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in rats and rabbits at doses up to 30 times the human dose (based on 50 kg weight) and have shown no evidence of impaired fertility or harm to the animal fetus. There are, however, no adequate and well-controlled studies with CANTIL in pregnant women. Because animal reproduction studies are not always predictive of human response, CANTIL should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mepenzolate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mepenzolate during labor and delivery.
Nursing Mothers
- It is not known whether CANTIL is secreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when CANTIL is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established. Studies in newborn animals (rats) show that younger animals are more sensitive to the toxic effects of CANTIL than are older animals.
Geriatic Use
- Clinical studies of CANTIL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
- CANTIL is contraindicated in intestinal atony of the elderly. CANTIL should be used with caution in the elderly.
Gender
There is no FDA guidance on the use of Mepenzolate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mepenzolate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mepenzolate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mepenzolate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mepenzolate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mepenzolate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Mepenzolate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Mepenzolate and IV administrations.
Overdosage
Signs and Symptoms
- The signs and symptoms of overdosage are headache; nausea; vomiting; blurred vision; dilated pupils; hot, dry skin; dizziness; dryness of the mouth; difficulty in swallowing; and CNS stimulation. A curare-like action may occur (i.e., neuromuscular blockade leading to muscular weakness and possible paralysis).
Oral LD50
- The oral LD50 is greater than 750 mg/kg in mice and greater than 1000 mg/kg in rats.
Maximum Human Dose Recorded
- The maximum human dose recorded is 375 to 500 mg in a 4-year-old child (no adverse effects reported) and 500 to 750 mg in a 30-year-old adult (resulted in death).
Dialysis
- It is not known if the drug is dialyzable.
Treatment
- Treatment should consist of gastric lavage, emetics, and activated charcoal. Sedatives (e.g., short-acting barbiturates, benzodiazepines) may be used for management of overt signs of excitement. If indicated, an appropriate parenteral cholinergic agent may be used as an antidote.
Pharmacology
| |
Mepenzolate
| |
| Systematic (IUPAC) name | |
| 3-(2-hydroxy-2,2-diphenylacetoxy)-1,1-dimethylpiperidinium | |
| Identifiers | |
| CAS number | |
| ATC code | A03 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox+ |
| Mol. mass | 340.44 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- CANTIL diminishes gastric acid and pepsin secretion. CANTIL also suppresses spontaneous contractions of the colon. Pharmacologically, it is a post-ganglionic parasympathetic inhibitor.
Structure
- CANTIL tablets for oral administration contain 25 mg mepenzolate bromide USP. The anticholinergic agent mepenzolate bromide USP chemically is 3-[(hydroxydiphenylacetyl)oxy]-1,1-dimethylpiperidinium bromide and has the following structure:

Pharmacodynamics
There is limited information regarding Mepenzolate Pharmacodynamics in the drug label.
Pharmacokinetics
- Radiotracer studies in which CANTIL-14C was used in animals and humans indicate the absorption following oral administration, as with other quaternary ammonium compounds, is low. Between 3 and 22% of an orally administered dose is excreted in the urine over a 5-day period, with the majority of the radioactivity appearing on Day 1. The remainder appears in the next 5 days in the feces and presumably has not been absorbed.
Nonclinical Toxicology
There is limited information regarding Mepenzolate Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Mepenzolate Clinical Studies in the drug label.
How Supplied
25 mg mepenzolate bromide, compressed yellow tablets debossed MERRELL 37.
NDC 0068-0037-01: bottles of 100
Storage
- Keep tightly closed. Store at room temperature, preferably below 86°F.
- Protect from excessive heat. Dispense in tight containers with child-resistant closure.
Images
Drug Images
{{#ask: Page Name::Mepenzolate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 25MG TABLETS BOTTLE LABEL
NDC 0068-0037-01
Cantil® 25mg
mepenzolate bromide USP
100 Tablets
sanofi aventis

{{#ask: Label Page::Mepenzolate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- CANTIL may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness, such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug.
Precautions with Alcohol
Alcohol-Mepenzolate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Cantil
Look-Alike Drug Names
There is limited information regarding Mepenzolate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.